Abstract
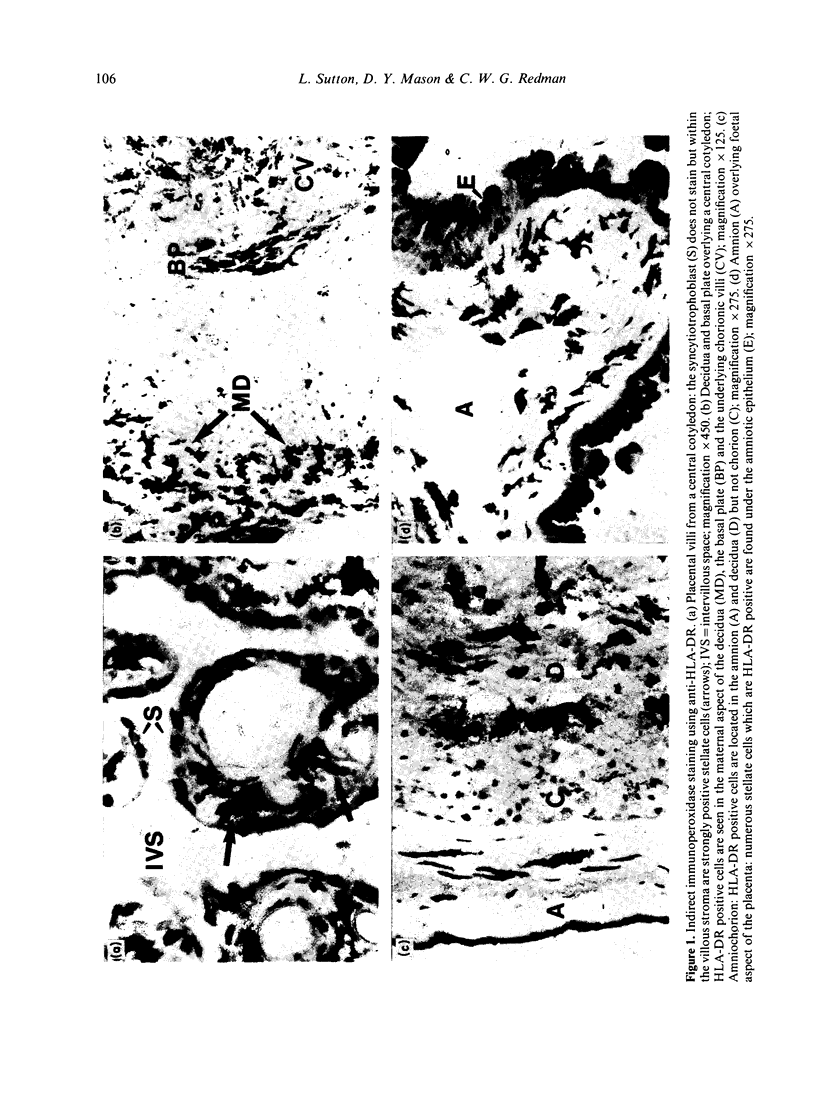
A population of heterogeneous HLA-DR positive cells has been identified in the human placenta and decidua using immunochemical and histochemical methods. These cells are found in three areas: the subepithelial layer of the amnion, the decidua, and more sparsely within the chorionic villous stroma. In addition to HLA-A, -B and -C antigens, they also express the leucocyte-common antigen, indicating their origin from bone marrow precursors. The majority have a characteristic stellate shape with many cytoplasmic processes. In the villous stroma these stellate cells can be distinguished from the Hofbauer cells (placental macrophages) by their morphology, stronger expression of HLA-DR and lack of lysosomal enzyme activity. In the amnion and decidua they cannot be clearly distinguished from tissue macrophages. By using monoclonal antibodies specific for foetal or maternal HLA-A or -B allotypes, the HLA-DR positive cells in the chorionic villi and the amnion have been shown to be foetal in origin. In contrast, most of the HLA-DR positive cells in the decidua are maternal; a few adjacent to the basal plate are foetal. The preponderance of these cells in those areas of the placenta where foetal and maternal tissues are in close proximity is striking. The possibility that some of the cells are equivalent to the dendritic cells that have been described in other tissues is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi M., Akle C. A., McColl I., Fensom A. H., Tansley L., Connolly P., Hsi B. L., Faulk W. P., Travers P., Bodmer W. F. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982 Jan 28;295(5847):325–327. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- Breard J., Fuks A., Friedman S. M., Schlossman S. F., Chess L. The role of p23,30-bearing human macrophages in antigen-induced T lymphocyte responses. Cell Immunol. 1979 Jun;45(1):108–119. doi: 10.1016/0008-8749(79)90366-6. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Ellis S. A., Taylor C., McMichael A. Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum Immunol. 1982 Aug;5(1):49–59. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- Enders A. C., King B. F. The cytology of Hofbauer cells. Anat Rec. 1970 Jun;167(2):231–236. doi: 10.1002/ar.1091670211. [DOI] [PubMed] [Google Scholar]
- Fox H., Kharkongor N. F. Enzyme histochemistry of the Hofbauer cells of the human placenta. J Obstet Gynaecol Br Commonw. 1969 Oct;76(10):918–921. doi: 10.1111/j.1471-0528.1969.tb15730.x. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Barnstable C. J., Bodmer W. F., Snary D., Crumpton M. J. Expression of HLA system antigens on placenta. Transplantation. 1976 Dec;22(6):595–603. doi: 10.1097/00007890-197612000-00009. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fuggle S. V., Williams K. A., Fabre J. W., Ting A., Morris P. J. Localization of HLA-ABC and DR antigens in human kidney. Transplantation. 1981 Jun;31(6):428–433. doi: 10.1097/00007890-198106000-00005. [DOI] [PubMed] [Google Scholar]
- Janossy G., Thomas J. A., Habeshaw J. A. Immunofluorescence analysis of normal and malignant lymphoid tissues with selected combination of antisera. J Histochem Cytochem. 1980 Nov;28(11):1207–1214. doi: 10.1177/28.11.7000889. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Malmnäs Tjernlund U. K., Kabelitz D., Wigren A. Appearance of anti-HLA-DR-reactive cells in normal and rheumatoid synovial tissue. Scand J Immunol. 1981 Aug;14(2):183–192. doi: 10.1111/j.1365-3083.1981.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Tjernlund U., Forsum U., Peterson P. A. Epidermal Langerhans cells express Ia antigens. Nature. 1977 Jul 21;268(5617):248–250. doi: 10.1038/268248a0. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Trägårdh L., Lindblom J. B., Peterson P. A. Reactivity of a rabbit antiserum against highly purified HLA-DR antigens. Scand J Immunol. 1978 Mar;7(3):199–208. doi: 10.1111/j.1365-3083.1978.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., O'Brien J. P., Beyer C. F., Bowers W. E. Rat dendritic cells function as accessory cells and control the production of a soluble factor required for mitogenic responses of T lymphocytes. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5414–5418. doi: 10.1073/pnas.77.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Parham P., Rust N., Brodsky F. A monoclonal antibody that recognizes an antigenic determinant shared by HLA A2 and B17. Hum Immunol. 1980 Sep;1(2):121–129. doi: 10.1016/0198-8859(80)90099-3. [DOI] [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Sunderland C. A., Allington M. J., Stein H., Mason D. Y. The value of immunohistological screening in the production of monoclonal antibodies. J Immunol Methods. 1982;50(2):145–160. doi: 10.1016/0022-1759(82)90221-6. [DOI] [PubMed] [Google Scholar]
- Niederhuber J. E. The role of I region gene products in macrophage - T lymphocyte interaction. Immunol Rev. 1978;40:28–52. doi: 10.1111/j.1600-065x.1978.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980 May 1;151(5):1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Parker J. W., Frelinger J. A., O'Brien R. L. Characterization of responding cells in oxidative mitogen stimulation. II. Identification of an Ia-bearing adherent accessory cell. J Immunol. 1980 Jun;124(6):2700–2707. [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- Rowden G., Lewis M. G., Sullivan A. K. Ia antigen expression on human epidermal Langerhans cells. Nature. 1977 Jul 21;268(5617):247–248. doi: 10.1038/268247a0. [DOI] [PubMed] [Google Scholar]
- Scott H., Brandtzaeg P., Hirschberg H., Solheim B. G., Thorsby E. Vascular and renal distribution of HLA--DR-like antigens. Tissue Antigens. 1981 Sep;18(3):195–202. doi: 10.1111/j.1399-0039.1981.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland C. A., Naiem M., Mason D. Y., Redman C. W., Stirrat G. M. The expression of major histocompatibility antigens by human chorionic villi. J Reprod Immunol. 1981 Dec;3(6):323–331. doi: 10.1016/0165-0378(81)90048-6. [DOI] [PubMed] [Google Scholar]
- Sunshine G. H., Katz D. R., Feldmann M. Dendritic cells induce T cell proliferation to synthetic antigens under Ir gene control. J Exp Med. 1980 Dec 1;152(6):1817–1822. doi: 10.1084/jem.152.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet P. Human Ia-type alloantigens: methods of detection, aspects of chemistry and biology, markers for disease states. Transplant Rev. 1976;30:271–298. doi: 10.1111/j.1600-065x.1976.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yamashita U., Shevach E. M. The expression of Ia antigens on immunocompetent cells in the guinea pig. II. Ia antigens on macrophages. J Immunol. 1977 Nov;119(5):1584–1588. [PubMed] [Google Scholar]