Abstract
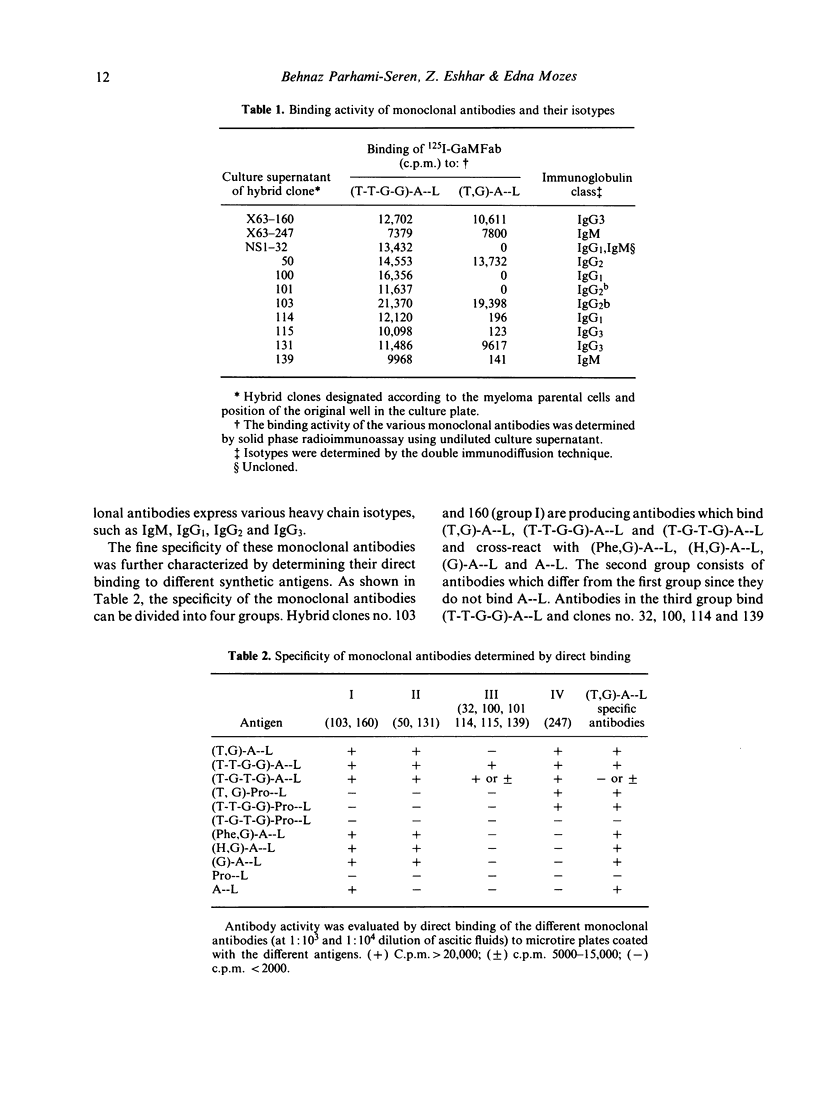
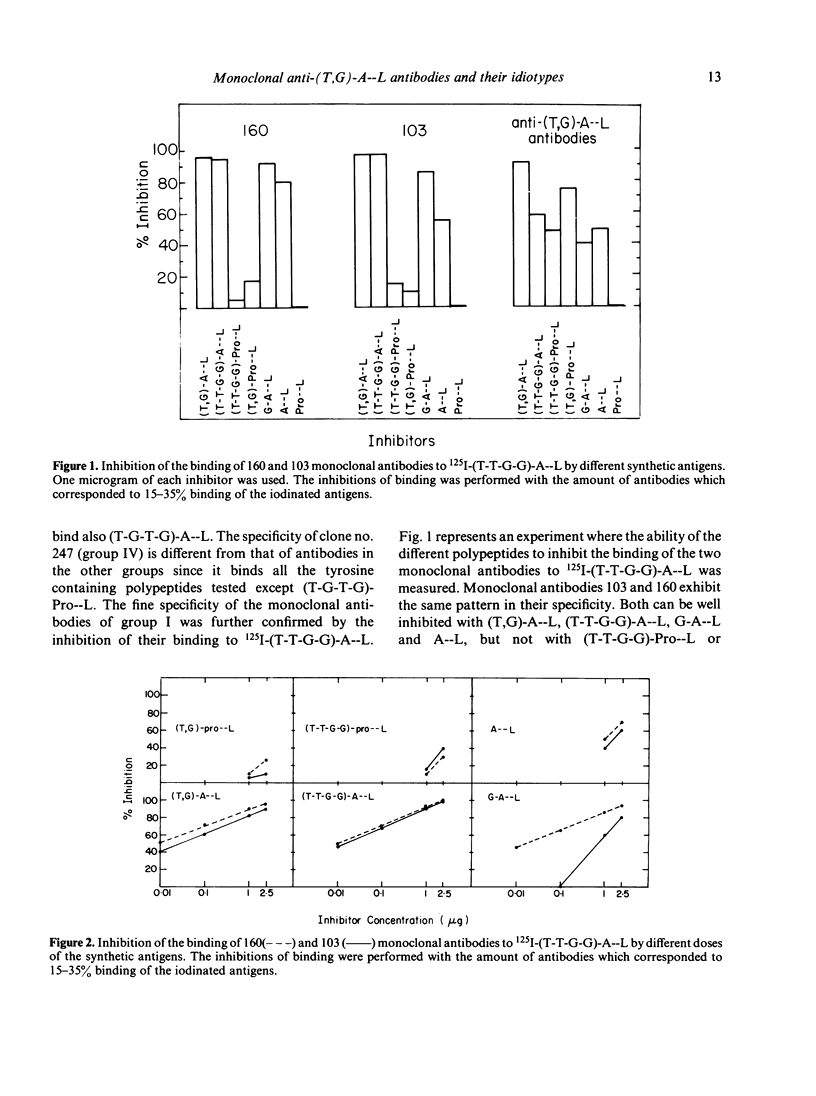
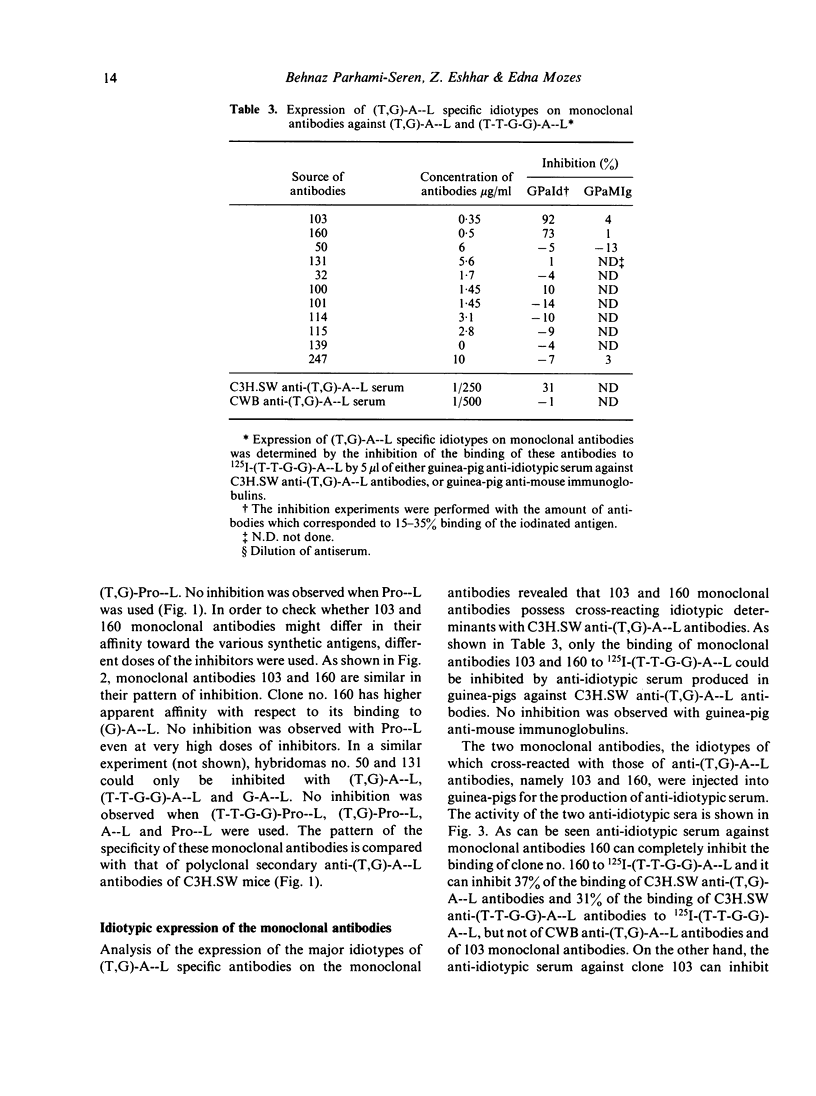
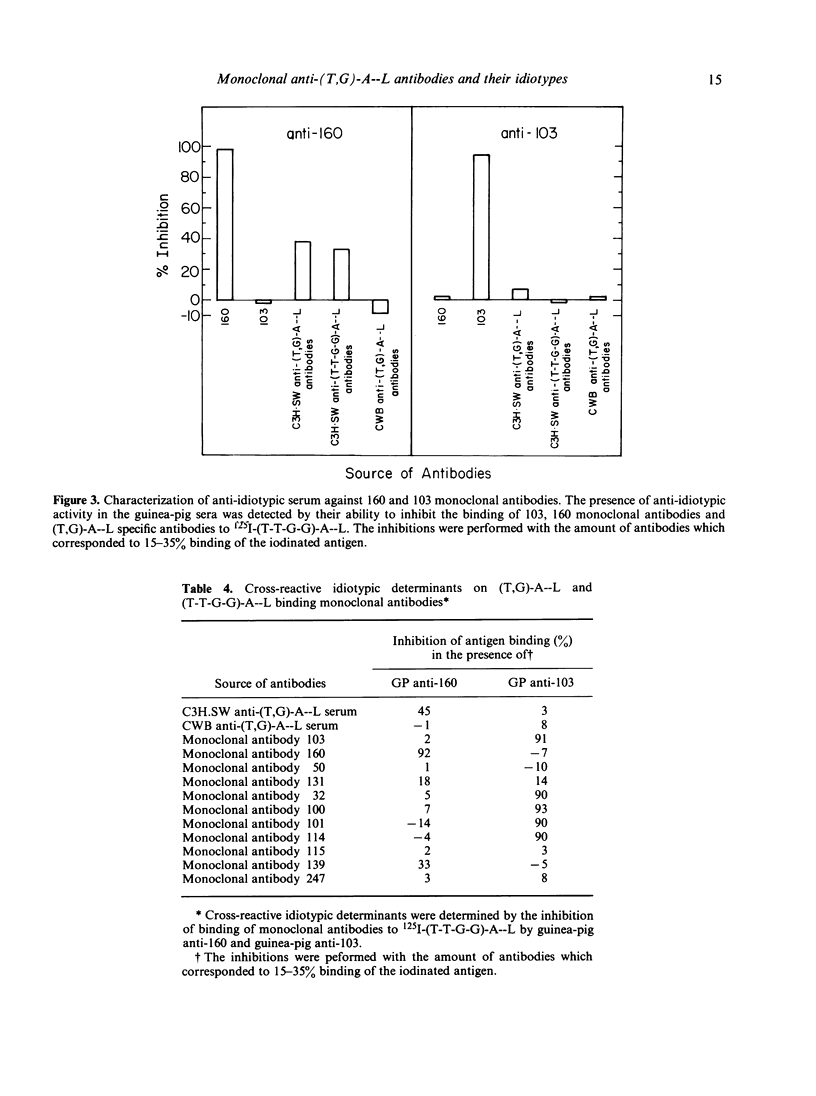
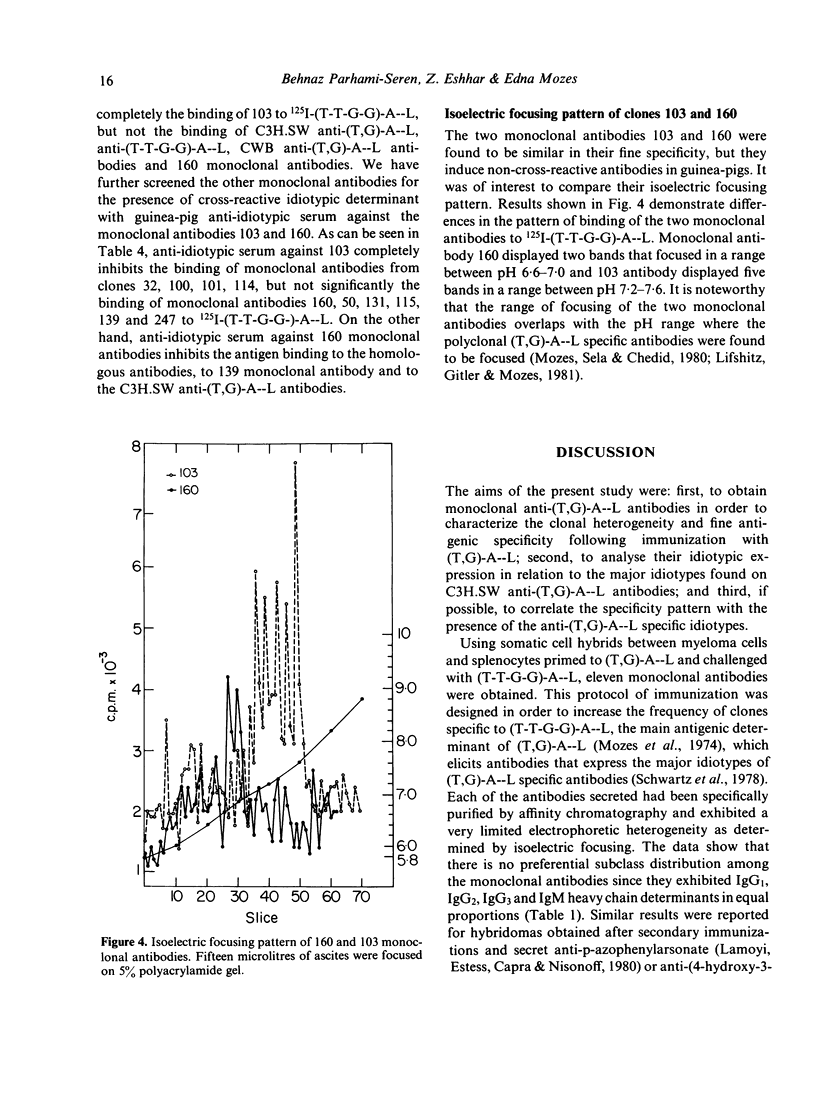
In order to study the repertoire of poly(Tyr,Glu)-poly(DLAla)--poly(Lys) [(T,G)-A--L] specific antibodies, monoclonal antibodies were prepared by fusing myeloma cells with spleen cells from C3H.SW mice immunized with (T,G)-A--L and boosted with (Tyr-Tyr-Glu-Glu)-poly(DLAla)--poly(Lys)](T-T-G-G)-A--L]. Eleven clones which secreted homogeneous antibodies were obtained. In general, two families of monoclonal antibodies were detected: those which bind exclusively (T-T-G-G)-A--L and those which bind both (T-T-G-G)-A--L and (T,G)-A--L. Analysis for idiotypic expression revealed that only two antibodies (clones no. 103 and 160), which were found to be similar in their fine specificity, cross-reacted with antibodies against the major idiotypes of (T,G)A--L specific antibodies. Guinea-pig antibodies against clone no. 160 reacted with the polyclonal (T,G)-A--L specific antibodies, whereas antibodies against 103 monoclonal antibodies did not react with C3H.SW anti-(T,G)-A--L antibodies, but did cross-react with four other monoclonal antibodies. It appears that the idiotypic determinants expressed on polyclonal (T,G)-A--L specific antibodies are heterogeneous, and consist of at least two serologically different idiotypes detected by clones no. 103 and 160.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Coutinho A., Melchers F. Absolute frequencies of lipopolysaccharide-reactive B cells producing A5A idiotype in unprimed, streptococcal A carbohydrate-primed, anti-A5A idiotype-sensitized and anti-A5A idiotype-suppressed A/J mice. J Exp Med. 1977 Nov 1;146(5):1436–1449. doi: 10.1084/jem.146.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z., Ofarim M., Waks T. Generation of hybridomas secreting murine reaginic antibodies of anti-DNP specificity. J Immunol. 1980 Feb;124(2):775–780. [PubMed] [Google Scholar]
- Eshhar Z., Strassmann G., Waks T., Mozes E. In vitro and in vivo induction of effector T cells mediating DTH responses to a protein and a synthetic polypeptide antigen. Cell Immunol. 1979 Oct;47(2):378–389. doi: 10.1016/0008-8749(79)90347-2. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Sela M. Antigenicity of some new synthetic polypeptides and polypeptidyl gelatins. Biochem J. 1964 Dec;93(3):566–572. doi: 10.1042/bj0930566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R. Standardization of the chloramine-T method of protein iodination. Proc Soc Exp Biol Med. 1970 Mar;133(3):989–992. doi: 10.3181/00379727-133-34611. [DOI] [PubMed] [Google Scholar]
- Jaton J. C., Sela M. Role of optical configuration in the immunogenicity and specificity of synthetic antigens derived from multichain polyproline. J Biol Chem. 1968 Nov 10;243(21):5616–5626. [PubMed] [Google Scholar]
- Ju S. T., Pierres M., Germain R. N., Benacerraf B., Dorf M. E. Idiotypic analysis of anti-GAT antibodies. VII. Common idiotype on hybridoma antibodies to poly(Glu60 Ala40) J Immunol. 1980 Sep;125(3):1230–1236. [PubMed] [Google Scholar]
- Ju S. T., Pierres M., Waltenbaugh C., Germain R. N., Benacerraf B., Dorf M. E. Idiotypic analysis of monoclonal antibodies to poly(Glu60Ala30Tyr10). Proc Natl Acad Sci U S A. 1979 Jun;76(6):2942–2946. doi: 10.1073/pnas.76.6.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoyi E., Estess P., Capra J. D., Nisonoff A. Heterogeneity of an intrastrain cross-reactive idiotype associated with anti-p-azophenylarsonate antibodies of A/J mice. J Immunol. 1980 Jun;124(6):2834–2840. [PubMed] [Google Scholar]
- Lifshitz R., Gitler C., Mozes E. Liposomes as immunological adjuvants in eliciting antibodies specific to the synthetic polypeptide poly(LTyr, LGlu)-poly(DLAla)--(LLys) with high frequency of site-associated idiotypic determinants. Eur J Immunol. 1981 May;11(5):398–404. doi: 10.1002/eji.1830110510. [DOI] [PubMed] [Google Scholar]
- Lifshitz R., Schwartz M., Mozes E. Linkage of murine (T,G)-A--L-specific idiotypic determinants to the heavy chain constant region allotypic markers. Immunogenetics. 1980;11(2):191–198. doi: 10.1007/BF01567784. [DOI] [PubMed] [Google Scholar]
- Lifshitz R., Schwartz M., Mozes E. Specificity and crossreactivity of idiotypes of murine antibodies induced by poly(Tyr,Glu)-poly(DLAla)-poly(Lys) and poly(Phe,Glu)-poly(DLAla)-poly(Lys). Proc Natl Acad Sci U S A. 1978 Oct;75(10):5118–5121. doi: 10.1073/pnas.75.10.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Chinitz A. Genetic control of the antibody response: relationship between immune response and histocompatibility (H-2) type. Science. 1969 Mar 14;163(3872):1207–1208. doi: 10.1126/science.163.3872.1207. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Sela M. Genetic control of the antibody response. I. Demonstration of determinant-specific differences in response to synthetic polypeptide antigens in two strains of inbred mice. J Exp Med. 1965 Sep 1;122(3):517–531. doi: 10.1084/jem.122.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D. W., Furman A., Miller A., Sercarz E. E. Idiotypic repertoire of anti-hen eggwhite lysozyme antibodies probed with hybridomas. Selection after immunization of an IdX marker common to antibodies of distinct epitope specificity. J Exp Med. 1981 Sep 1;154(3):701–712. doi: 10.1084/jem.154.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D. W., Miller A., Sercarz E. E. Sharing of an idiotypic marker by monoclonal antibodies specific for distinct regions of hen lysozyme. Nature. 1980 Oct 9;287(5782):540–542. doi: 10.1038/287540a0. [DOI] [PubMed] [Google Scholar]
- Mozes E., Schwartz M., Sela M. Antibody response of inbred mouse strains to ordered tetrapeptides of tyrosine and glutamic acid attached to multichain polyalanine or polyproline. Tyr-Tyr-Glu-Glu is a major determinant of the random poly-(Tyr, Glu)-polyDLAla--polyLys. J Exp Med. 1974 Aug 1;140(2):349–355. doi: 10.1084/jem.140.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes E., Sela M., Chedid L. Efficient genetically controlled formation of antibody to a synthetic antigen [poly(LTyr, LGlu)-poly(DLAla)- -poly(LLys)] covalently bound to a synthetic adjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine). Proc Natl Acad Sci U S A. 1980 Aug;77(8):4933–4937. doi: 10.1073/pnas.77.8.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes E., Shearer G. M. Genetic control of immune responses. Curr Top Microbiol Immunol. 1972;59:167–200. doi: 10.1007/978-3-642-65444-2_6. [DOI] [PubMed] [Google Scholar]
- Pierres M., Ju S. T., Waltenbaugh C., Dorf M. E., Benacerraf B., Germain R. N. Fine specificity of antibodies to poly(Glu60Ala30Tyr10) produced by hybrid cell lines. Proc Natl Acad Sci U S A. 1979 May;76(5):2425–2429. doi: 10.1073/pnas.76.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- SELA M., FUCHS S., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. Biochem J. 1962 Oct;85:223–235. doi: 10.1042/bj0850223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Lifshitz R., Givol D., Mozes E., Haimovich J. Cross-reactive idiotypic determinants on murine anti-(T,G)-A--L antibodies. J Immunol. 1978 Aug;121(2):421–426. [PubMed] [Google Scholar]


