Abstract
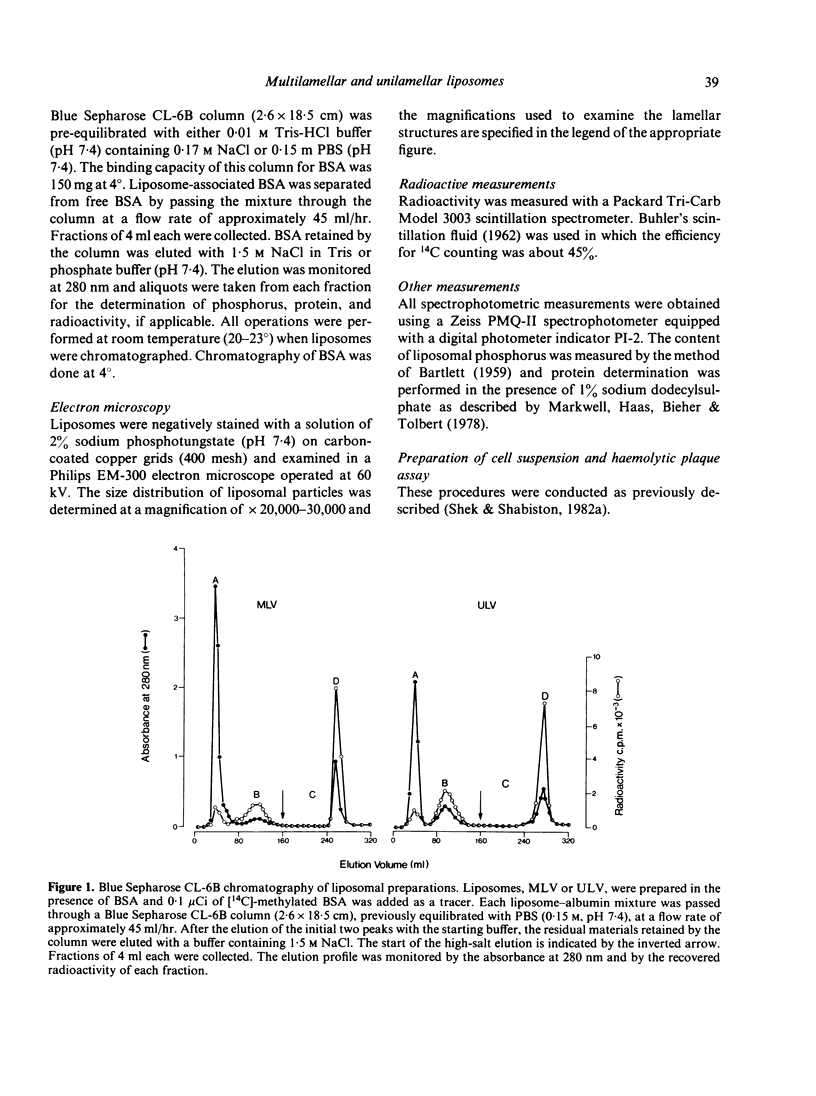
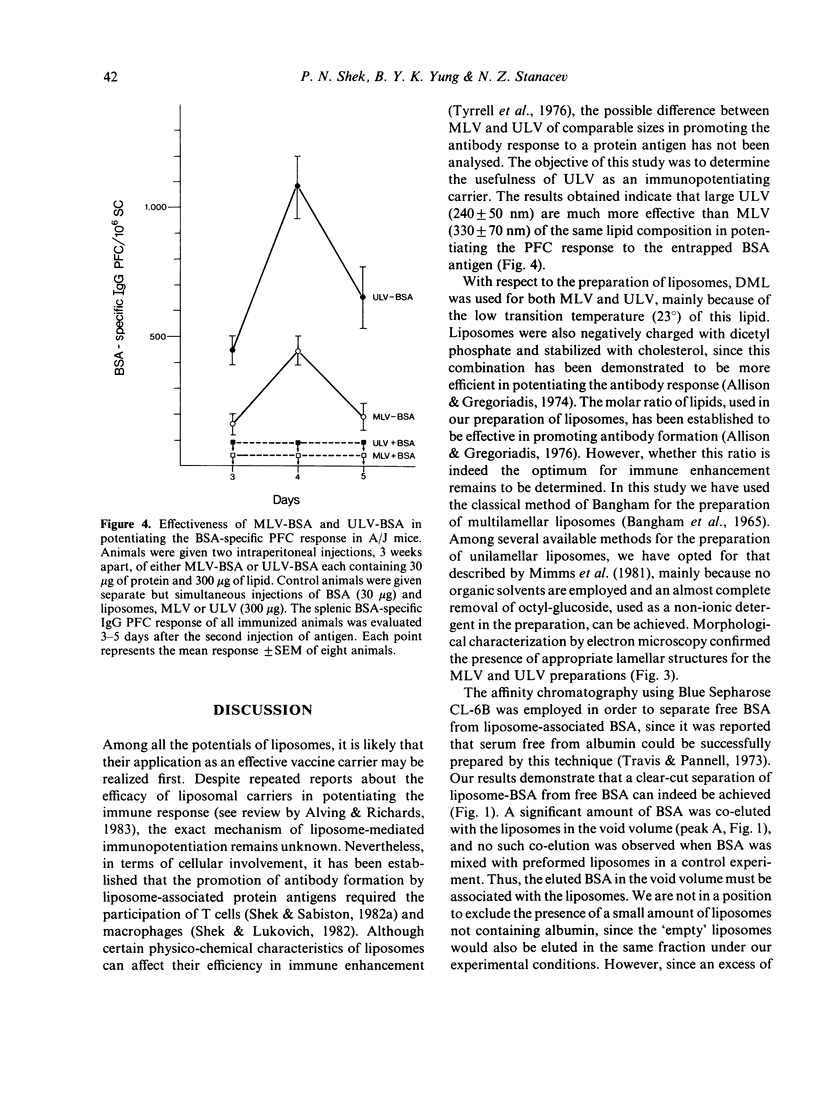
The effectiveness of two liposomal preparations, multilamellar vesicles (MLV) and unilamellar vesicles (ULV), in enhancing specific antibody formation was compared in this study. The MLV and ULV, prepared in the presence of bovine serum albumin (BSA), were composed of dimyristoyl-lecithin, cholesterol and dicetyl phosphate in a molar ratio of 7:2:1. Excess free BSA was separated from liposome-associated BSA by Blue Sepharose CL-6B column chromatography. The presence of appropriate lamellar structures for each liposome preparation was demonstrated by electron microscopy. The simultaneous injection of control mice with free BSA and 'empty' MLV or ULV failed to elicit a BSA-specific plaque-forming cell (PFC) response. In contrast, animals injected with liposome-associated BSA (MLV-BSA or ULV-BSA) generated a vigorous PFC response; the magnitude of the response induced by BSA entrapped in unilamellar vesicles was significantly higher than that in multilamellar vesicles. These results suggest that the lamellar arrangement of liposomal vesicles may play a role in affecting the magnitude of the potentiated antibody response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BUHLER D. R. A simple scintillation counting technique for assaying C1402 in a Warburg flask. Anal Biochem. 1962 Nov;4:413–417. doi: 10.1016/0003-2697(62)90143-4. [DOI] [PubMed] [Google Scholar]
- Bangham A. D., Standish M. M., Watkins J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965 Aug;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- Denes A. S., Stanacev N. Z. Spin-label study of the relation between enzymatic activity and lipid-protein organization in reconstituted cytochrome c oxidase. Can J Biochem. 1978 Sep;56(9):905–915. doi: 10.1139/o78-140. [DOI] [PubMed] [Google Scholar]
- Denes A. S., Stanacev N. Z. Thermally induced changes in reconstituted and membranous cytochrome c oxidase. Can J Biochem. 1979 Mar;57(3):238–249. doi: 10.1139/o79-030. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Allison A. C. Entrapment of proteins in liposomes prevents allergic reactions in pre-immunised mice. FEBS Lett. 1974 Sep 1;45(1):71–74. doi: 10.1016/0014-5793(74)80813-6. [DOI] [PubMed] [Google Scholar]
- Heath T. D., Edwards D. C., Ryman B. E. The adjuvant properties of liposomes. Biochem Soc Trans. 1976;4(1):129–133. doi: 10.1042/bst0040129. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Stamp D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem Biophys Res Commun. 1975 Apr 7;63(3):651–658. doi: 10.1016/s0006-291x(75)80433-5. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mimms L. T., Zampighi G., Nozaki Y., Tanford C., Reynolds J. A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Ryman B. E., Tyrrell D. A. Liposomes--bags of potential. Essays Biochem. 1980;16:49–98. [PubMed] [Google Scholar]
- Shek P. N., Lukovich S. The role of macrophages in promoting the antibody response mediated by liposome-associated protein antigens. Immunol Lett. 1982;5(6):305–309. doi: 10.1016/0165-2478(82)90118-3. [DOI] [PubMed] [Google Scholar]
- Shek P. N., Sabiston B. H. Immune response mediated by liposome-associated protein antigens. I. Potentiation of the plaque-forming cell response. Immunology. 1982 Feb;45(2):349–356. [PMC free article] [PubMed] [Google Scholar]
- Shek P. N., Sabiston B. H. Immune response mediated by liposome-associated protein antigens. II. Comparison of the effectiveness of vesicle-entrapped and surface-associated antigen in immunopotentiation. Immunology. 1982 Dec;47(4):627–632. [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Heath T. D., Colley C. M., Ryman B. E. New aspects of liposomes. Biochim Biophys Acta. 1976 Dec 14;457(3-4):259–302. doi: 10.1016/0304-4157(76)90002-2. [DOI] [PubMed] [Google Scholar]
- Vaughan D. J., Breckenridge W. C., Stanacev N. Z. Reconstitution of lipoproteins. I. Lipid-protein interaction of high density apoproteins, purified apoA-I and apoA-II with dimyristoyl-lecithin and dimyristoyl-lecithin:cholesterol vesicles studied by isomeric spin-labelled lecithins. Can J Biochem. 1980 Jul;58(7):581–591. doi: 10.1139/o80-080. [DOI] [PubMed] [Google Scholar]
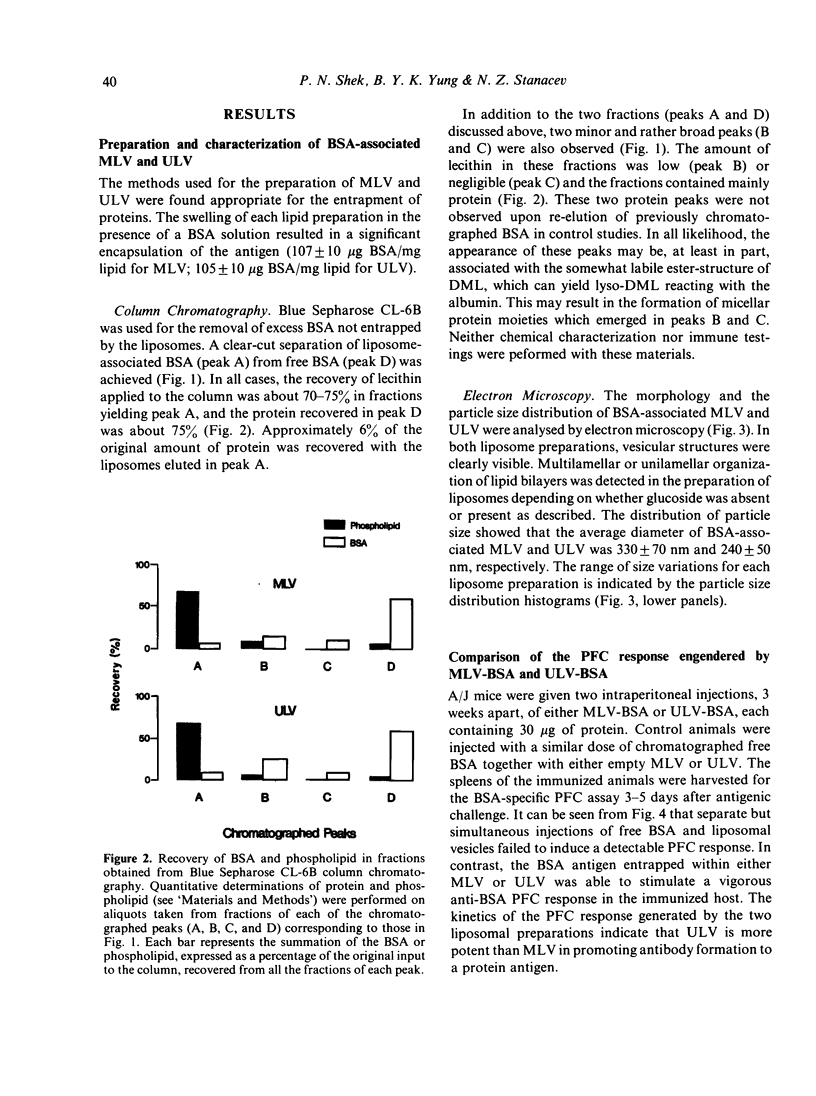
- Vaughan D. J., Breckenridge W. C., Stanacev N. Z. Reconstitution of lipoproteins. II. Lipid-protein interaction between dimyristoyl-lecithin and dimyristoyl-lecithin:cholesterol vesicles and purified apolipoprotein C-I and C-III2 studied by isomeric spin-labelled lecithins. Can J Biochem. 1980 Jul;58(7):592–598. doi: 10.1139/o80-081. [DOI] [PubMed] [Google Scholar]