Abstract
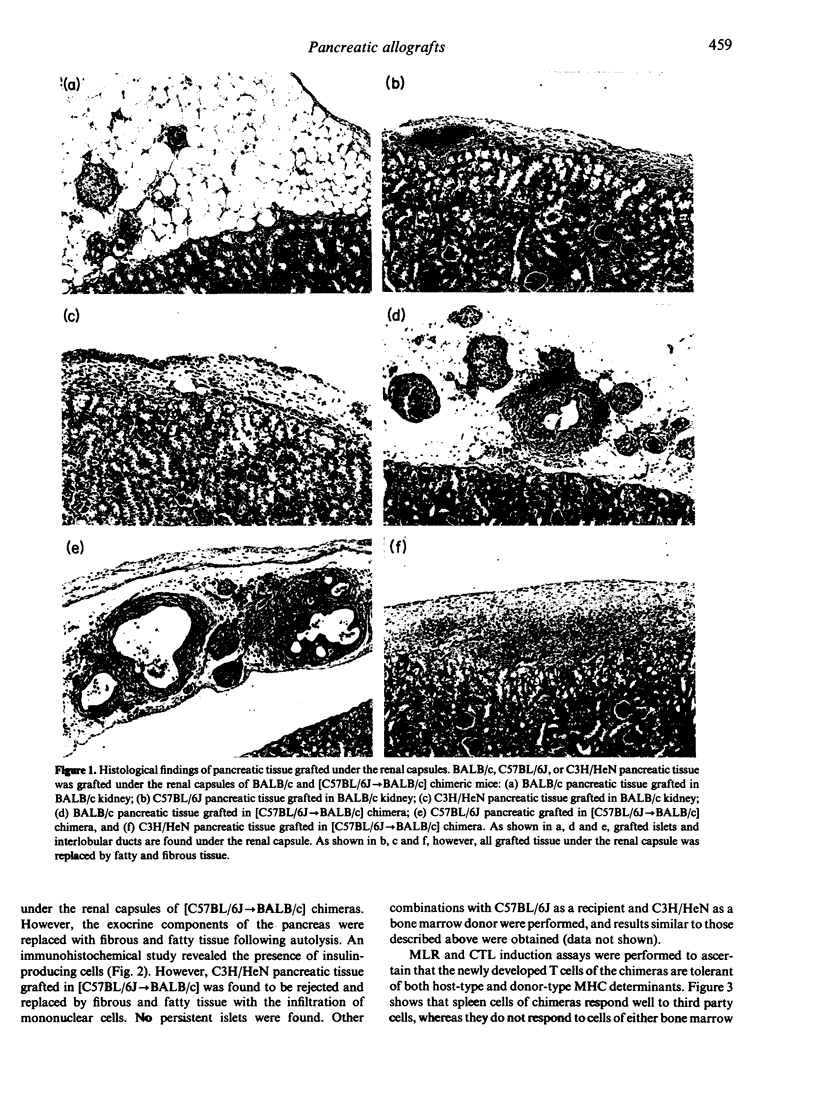
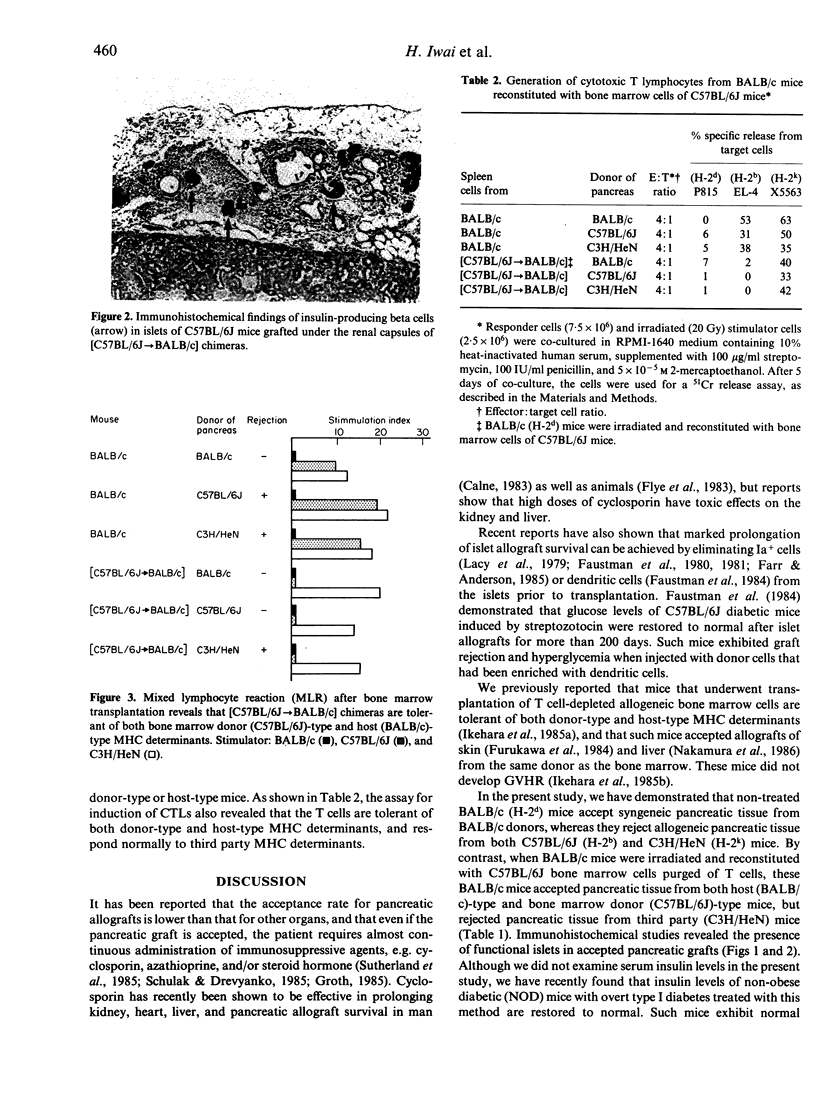
We have established a new method for pancreatic allografts in mice by combining pancreatic transplantation with allogeneic bone marrow transplantation. In this approach, we first transplanted bone marrow to induce tolerance to both donor-type and host-type major histocompatibility complex (MHC) determinants. Pancreatic tissue from the same mouse strain as bone marrow donor was then grafted under the renal capsule. Acceptance of the grafts was confirmed by histopathological and immunohistochemical techniques. BALB/c mice reconstituted with C57BL/6J bone marrow cells accepted pancreatic tissue from both bone marrow donor (C57BL/6J)-type and host (BALB/c)-type mice. An immunohistochemical study revealed the presence of functional islets under the renal capsules. Assays for both mixed lymphocyte reaction (MLR) and induction of cytotoxic T lymphocytes indicated that the newly developed T cells are tolerant of both donor (stem cell)-type and host-type MHC determinants. By contrast, the T cells of these chimeras showed a significant responsiveness to third party MHC determinants. These findings suggest that pancreatic allografts combined with bone marrow transplantation may become a viable strategy for the treatment of patients with diabetes or patients who have undergone pancreatectomy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dresden M. H., Sung C. K., Deelder A. M. A monoclonal antibody from infected mice to a Schistosoma mansoni egg proteinase. J Immunol. 1983 Jan;130(1):1–3. [PubMed] [Google Scholar]
- Farr A. G., Anderson S. K. In situ ultrastructural demonstration of cells bearing Ia antigens in the murine pancreas. Diabetes. 1985 Oct;34(10):987–990. doi: 10.2337/diab.34.10.987. [DOI] [PubMed] [Google Scholar]
- Faustman D. L., Steinman R. M., Gebel H. M., Hauptfeld V., Davie J. M., Lacy P. E. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3864–3868. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman D., Hauptfeld V., Davie J. M., Lacy P. E., Shreffler D. C. Murine pancreatic beta-cells express H-2K and H-2D but not Ia antigens. J Exp Med. 1980 Jun 1;151(6):1563–1568. doi: 10.1084/jem.151.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman D., Hauptfeld V., Lacy P., Davie J. Prolongation of murine islet allograft survival by pretreatment of islets with antibody directed to Ia determinants. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5156–5159. doi: 10.1073/pnas.78.8.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa F., Ikehara S., Tanaka H., Inoue S., Nakamura T., Hamashima Y. Fate of engrafted skin in thymic chimeras. Microbiol Immunol. 1984;28(9):1071–1076. doi: 10.1111/j.1348-0421.1984.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Good R. A., Kapoor N., Reisner Y. Bone marrow transplantation--an expanding approach to treatment of many diseases. Cell Immunol. 1983 Nov;82(1):36–54. doi: 10.1016/0008-8749(83)90139-9. [DOI] [PubMed] [Google Scholar]
- Ikehara S., Good R. A., Nakamura T., Sekita K., Inoue S., Oo M. M., Muso E., Ogawa K., Hamashima Y. Rationale for bone marrow transplantation in the treatment of autoimmune diseases. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2483–2487. doi: 10.1073/pnas.82.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Ohtsuki H., Good R. A., Asamoto H., Nakamura T., Sekita K., Muso E., Tochino Y., Ida T., Kuzuya H. Prevention of type I diabetes in nonobese diabetic mice by allogenic bone marrow transplantation. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7743–7747. doi: 10.1073/pnas.82.22.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindred B., Loor F. Activity of host-derived T cells which differentiate in nude mice grafted with co-isogenic or allogeneic thymuses. J Exp Med. 1974 May 1;139(5):1215–1227. doi: 10.1084/jem.139.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M., Finke E. H., Scharp D. W. Prolongation of islet allograft survival. Transplantation. 1979 Mar;27(3):171–174. doi: 10.1097/00007890-197903000-00006. [DOI] [PubMed] [Google Scholar]
- Mascret B., Maraninchi D., Gastaut J. A., Tubiana N., Sebahoun G., Horschowski N., Sainty D., Camerlo J., Lejeune C., Novakovitch G. Treatment of malignant lymphoma with high dose of chemo or chemoradiotherapy and bone marrow transplantation. Eur J Cancer Clin Oncol. 1986 Apr;22(4):461–471. doi: 10.1016/0277-5379(86)90113-6. [DOI] [PubMed] [Google Scholar]
- Müller-Ruchholtz W., Wottge H. U., Müller-Hermelink H. K. Bone marrow transplantation in rats across strong histocompatibility barriers by selective elimination of lymphoid cells in donor marrow. Transplant Proc. 1976 Dec;8(4):537–541. [PubMed] [Google Scholar]
- Nakamura T., Good R. A., Yasumizu R., Inoue S., Oo M. M., Hamashima Y., Ikehara S. Successful liver allografts in mice by combination with allogeneic bone marrow transplantation. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4529–4532. doi: 10.1073/pnas.83.12.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoé K., Yasumizu R., Geng L., Iwabuchi K., Ogasawara M., Kakinuma M., Okuyama H., Good R. A., Morikawa K. Analyses of Ia restriction specificity of helper T cells in H-2 subregion compatible bone marrow chimera in mice. Immunobiology. 1985 Feb;169(1):71–82. doi: 10.1016/S0171-2985(85)80055-3. [DOI] [PubMed] [Google Scholar]
- Organ transplantation. Pancreas. Transplant Proc. 1985 Feb;17(1 Pt 1):302–427. [PubMed] [Google Scholar]
- Seger R., Rogers K., Catty D. Differentiation of T cell precursors in nude mice. Rejection of heart grafts of thymus donor strain. Eur J Immunol. 1974 Jul;4(7):524–526. doi: 10.1002/eji.1830040716. [DOI] [PubMed] [Google Scholar]
- Storb R., Thomas E. D. Allogeneic bone-marrow transplantation. Immunol Rev. 1983;71:77–102. doi: 10.1111/j.1600-065x.1983.tb01069.x. [DOI] [PubMed] [Google Scholar]
- Yunis E. J., Fernandes G., Smith J., Good R. A. Long survival and immunologic reconstitution following transplantation with syngeneic or allogeneic fetal liver and neonatal spleen cells. Transplant Proc. 1976 Dec;8(4):521–525. [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Waterfield E., Kindred B., Welsh R. M., Callahan G., Pincetl P. Restriction specificities, alloreactivity, and allotolerance expressed by T cells from nude mice reconstituted with H-2-compatible or -incompatible thymus grafts. J Exp Med. 1980 Feb 1;151(2):376–399. doi: 10.1084/jem.151.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Sprent J., Nabholz M. Hemopoietic reconstitution obtained in F1 hybrids by grafting of parental marrow cells. Transplant Proc. 1976 Sep;8(3):355–358. [PubMed] [Google Scholar]




