Abstract
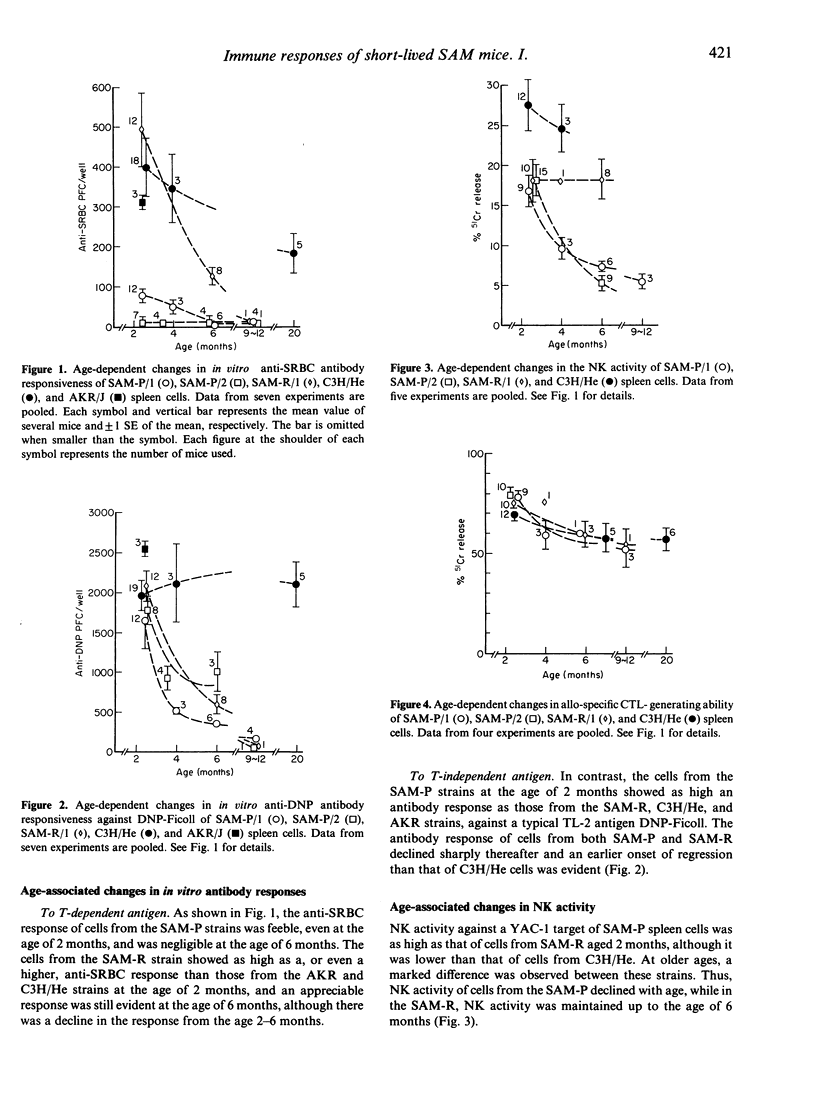
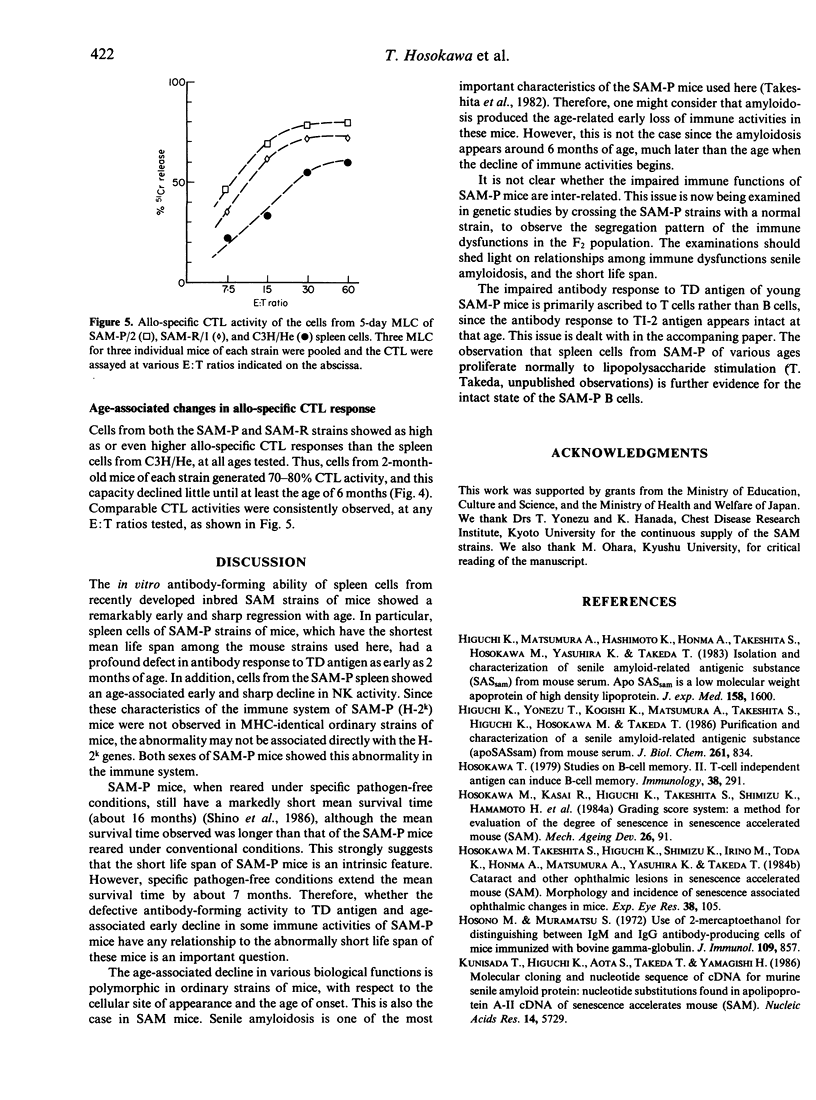
Using a cell culture system, age-associated changes in immune activities were investigated in newly developed, short-lived mouse strains. These SAM-P strains of mice (H-2k), which have a remarkably short life span (around 9 months) under conventional breeding conditions, showed an age-associated early decline in several immune functions, as compared to ordinary strains of AKR/J (H-2k) and C3H/He (H-2k) mice. Their antibody-forming capacity to T-independent antigen, DNP-Ficoll, and natural killer (NK) cell activity showed a markedly early onset of regression and a sharp decline from the level of control mice at 2 months of age. SAM-P strains of mice have a profound defect in antibody response to a T-dependent (TD) antigen, such as sheep red blood cells (SRBC), thus there was only a feeble antibody response to SRBC as early as the age of 2 months, and a negligible response at a later age. In contrast, the allo-specific cytotoxic T lymphocyte (CTL) response of the mice was as high as that of control mouse strains at 2 months of age and declined little until at least 6 months of age. The early age-related functional decline in the immune system of SAM-P mice suggests that these new inbred strains are appropriate models for investigating the age-related appearance of immune dysfunctions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Higuchi K., Matsumura A., Hashimoto K., Honma A., Takeshita S., Hosokawa M., Yasuhira K., Takeda T. Isolation and characterization of senile amyloid--related antigenic substance (SASSAM) from mouse serum. Apo SASSAM is a low molecular weight apoprotein of high density lipoprotein. J Exp Med. 1983 Nov 1;158(5):1600–1614. doi: 10.1084/jem.158.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa M., Kasai R., Higuchi K., Takeshita S., Shimizu K., Hamamoto H., Honma A., Irino M., Toda K., Matsumura A. Grading score system: a method for evaluation of the degree of senescence in senescence accelerated mouse (SAM). Mech Ageing Dev. 1984 Jul;26(1):91–102. doi: 10.1016/0047-6374(84)90168-4. [DOI] [PubMed] [Google Scholar]
- Hosokawa M., Takeshita S., Higuchi K., Shimizu K., Irino M., Toda K., Honma A., Matsumura A., Yasuhira K., Takeda T. Cataract and other ophthalmic lesions in senescence accelerated mouse (SAM). Morphology and incidence of senescence associated ophthalmic changes in mice. Exp Eye Res. 1984 Feb;38(2):105–114. doi: 10.1016/0014-4835(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Hosokawa T. Studies on B-cell memory. II. T-cell independent antigen can induce B-cell memory. Immunology. 1979 Oct;38(2):291–299. [PMC free article] [PubMed] [Google Scholar]
- Hosono M., Muramatsu S. Use of 2-mercaptoethanol for distinguishing between IgM and IgG antibody-producing cells of mice immunized with bovine globulin. J Immunol. 1972 Oct;109(4):857–863. [PubMed] [Google Scholar]
- Kunisada T., Higuchi K., Aota S., Takeda T., Yamagishi H. Molecular cloning and nucleotide sequence of cDNA for murine senile amyloid protein: nucleotide substitutions found in apolipoprotein A-II cDNA of senescence accelerated mouse (SAM). Nucleic Acids Res. 1986 Jul 25;14(14):5729–5740. doi: 10.1093/nar/14.14.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura A., Higuchi K., Shimizu K., Hosokawa M., Hashimoto K., Yasuhira K., Takeda T. A novel amyloid fibril protein isolated from senescence-accelerated mice. Lab Invest. 1982 Sep;47(3):270–275. [PubMed] [Google Scholar]
- Matsushita M., Tsuboyama T., Kasai R., Okumura H., Yamamuro T., Higuchi K., Higuchi K., Kohno A., Yonezu T., Utani A. Age-related changes in bone mass in the senescence-accelerated mouse (SAM). SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol. 1986 Nov;125(2):276–283. [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Takeda T., Hosokawa M., Takeshita S., Irino M., Higuchi K., Matsushita T., Tomita Y., Yasuhira K., Hamamoto H., Shimizu K. A new murine model of accelerated senescence. Mech Ageing Dev. 1981 Oct;17(2):183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Hosokawa M., Irino M., Higuchi K., Shimizu K., Yasuhira K., Takeda T. Spontaneous age-associated amyloidosis in senescence-accelerated mouse (SAM). Mech Ageing Dev. 1982 Sep;20(1):13–23. doi: 10.1016/0047-6374(82)90070-7. [DOI] [PubMed] [Google Scholar]


