Abstract
A transgene inserted in euchromatin exhibits mosaic expression when targeted by a fusion protein made of the DNA-binding domain of GAL4 and the heterochromatin-associated protein HP1. The silencing responds to the loss of a dose of the dominant modifiers of position-effect variegation Su(var)3-7 and Su(var)2-5, the locus encoding HP1. The genomic environs of the insertion site at 87C1 comprise the dispersed repetitive elements micropia and αγ. In the presence of the GAL4–HP1 chimera, the polytene chromosomes of this line form loops between the insertion site of the transgene and six other sections of chromosome 3R, as well as, rarely, with pericentric and telomeric heterochromatin. In contrast to the insertion site of the transgene at 87C, the six loop-forming sites in the euchromatic arm were each previously described as intercalary heterochromatin. Moreover, GAL4–HP1 tethering on one homologue trans-inactivates the reporter on the other. HP1, probably together with other partners, could thus facilitate the coalescence of dispersed middle repetitive sequences, and organize the heterochromatic structure responsible for the variegated silencing of nearby euchromatic genes.
Keywords: Drosophila/heterochromatin/HP1/position-effect variegation
Introduction
Position-effect variegation was discovered as mosaic phenotypes resulting from chromosomal rearrangements relocating euchromatic genes next to blocks of pericentric heterochromatin (Müller, 1930; Schultz, 1936). The frequency of inactivation varies with the dose of genetic modifiers (Spofford, 1976; Reuter and Spierer, 1992; Wallrath, 1998). Among them, the dominant suppressor of position-effect variegation Su(var)2-5 encodes the heterochromatin-associated protein HP1 (Eissenberg et al., 1990, 1992). The protein is associated with centromeric heterochromatin in interphase and mitotic chromosomes, and also with telomeres and some euchromatic sites (James et al., 1989; Kellum et al., 1995; Fanti et al., 1998a). Drosophila HP1 has homologues in a number of species and forms a protein family (reviewed by Eissenberg and Elgin, 2000). All members contain two conserved domains: the chromodomain and the chromo shadow domain, the latter being involved in interaction with protein partners (Aasland and Stewart, 1995; Koonin et al., 1995). In Drosophila, we have shown that HP1 interacts with another heterochromatin-associated modifier of variegation, Su(var)3-7 (Cléard et al., 1997; Delattre et al., 2000). Drosophila HP1 also interacts with origin recognition complex (ORC) proteins and co-localizes with the Arp4 protein in pericentric heterochromatin (Frankel et al., 1997; Pak et al., 1997). In mammalian cells, HP1 homologues are recruited by transcription intermediary factors, possibly to establish a heterochromatin-like complex (Le Douarin et al., 1996; Lehming et al., 1998; Seeler et al., 1998; Ryan et al., 1999). Transcriptional repression is observed in these mammalian cultured cells, but whether this is analogous to variegated silencing was not determined. In contrast, binding of HP1 seems necessary but not sufficient for silencing in flies (Fanti et al., 1998b). Mammalian HP1γ also interacts with the lamin B receptor, a nuclear membrane protein (Ye and Worman, 1996; Ye et al., 1997), and co-immunoprecipitates with Suv39h1, a homologue of the Drosophila suppressor of variegation Su(var)3-9 (Tschiersch et al., 1994; Aagaard et al., 1999). Mouse HP1 proteins interact with the p150 subunit of chromatin assembly factor 1 (CAF-1) (Murzina et al., 1999). Finally, human HP1 homologues seem to interact with the inner centromere protein (INCENP), a component of the mitotic chromosome (Ainsztein et al., 1998). These many observations suggest a role for HP1 in the control of transcription and replication, as well as in the establishment of heterochromatin-like repressive complexes and the organization of nuclear architecture.
To delineate the function of HP1 better, we have targeted a GAL4–HP1 fusion protein to ectopic sites in euchromatin, and we find that it induces variegated silencing of a flanking reporter gene in one of six insertion sites tested. In addition, in the presence of GAL4–HP1 we see formation of chromosomal loops between this site and regions of intercalary heterochromatin. Our observations give direct support to a model whereby repeated elements dispersed in the genome could recognize each other with the help of proteins such as HP1, and merge in a structure capable of silencing nearby genes.
Results
A euchromatic insertion of the reporter transgene Winkelried variegates when targeted by a GAL4–HP1 fusion protein
We addressed the question of whether genetic modifiers of position-effect variegation, which encode heterochromatin-associated proteins, induce silencing when targeted to a reporter gene inserted in euchromatin. To test the effect of these heterochromatin-associated proteins, we have built a reporter transgene for position-effect variegation, Winkelried, containing three binding sites for the yeast GAL4 transcriptional activator (Seum et al., 2000). The binding sites are inserted between the white and lacZ reporter genes, and are flanked by FRT sequences that allow their excision by crossing the transgenic line with an FLP-recombinase-producing strain. We have produced a number of transgenic lines of Winkelried (Seum et al., 2000). As expected, the lines with sites of insertion of the transgene in euchromatin do not variegate.
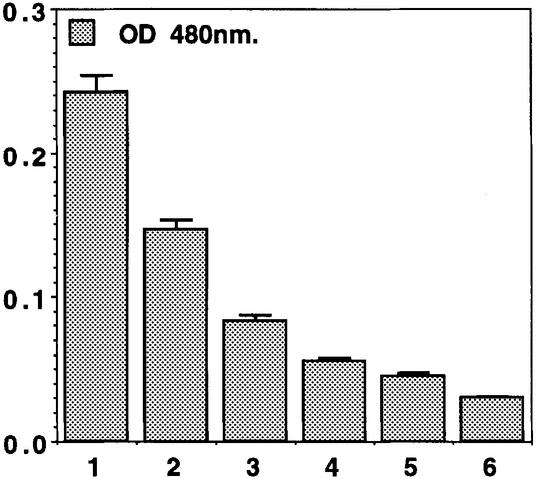
Nonetheless, we have combined these non-variegating lines with a transgene producing a heat-shock-inducible fusion protein made of the yeast GAL4 DNA-binding domain and most of the HP1 sequence. Surprisingly, one out of six lines with insertion of Winkelried in euchromatin, Wink-A7, presents a mosaic white phenotype in the eye when the HP1–GAL4 fusion protein is expressed (Figure 1). Variegation of Wink-A7 depends strictly on the presence of GAL4 binding sites, as verified with lines where the sites had been excised by the FRT/FLP system. In the presence of the GAL4 binding sites, an increase in dose of GAL4–HP1 augments silencing (Figures 1 and 2). To test whether the silencing of Wink-A7 induced by the GAL4–HP1 fusion protein uses the same mechanisms as position-effect variegation caused by pericentric heterochromatin, we have combined a GAL4–HP1/Wink-A7 (containing the GAL4 binding sites) with a loss of or an excess dose of two modifier loci known to affect ‘classical’ position-effect variegation. The loss of a dose of either the Su(var)3-7 or Su(var)2-5 gene does indeed suppress variegation of Wink-A7 (Figure 3). This response to modifiers indicates that the variegation of Wink-A7 induced in euchromatin by targeted HP1 uses components of classical heterochromatin-induced variegation. We also find that, in this context, an excess dose of either HP1 or Su(var)3-7 does not enhance variegation (Figure 3). These experiments also underline that HP1 needs to be targeted at the vicinity of miniwhite, because overexpression of a wild-type HP1, instead of GAL4–HP1, under heat shock promoter [HSHP1-83C in Eissenberg et al. (1992)] does not induce variegation of the miniwhite reporter gene in Wink-A7 (Figure 3).
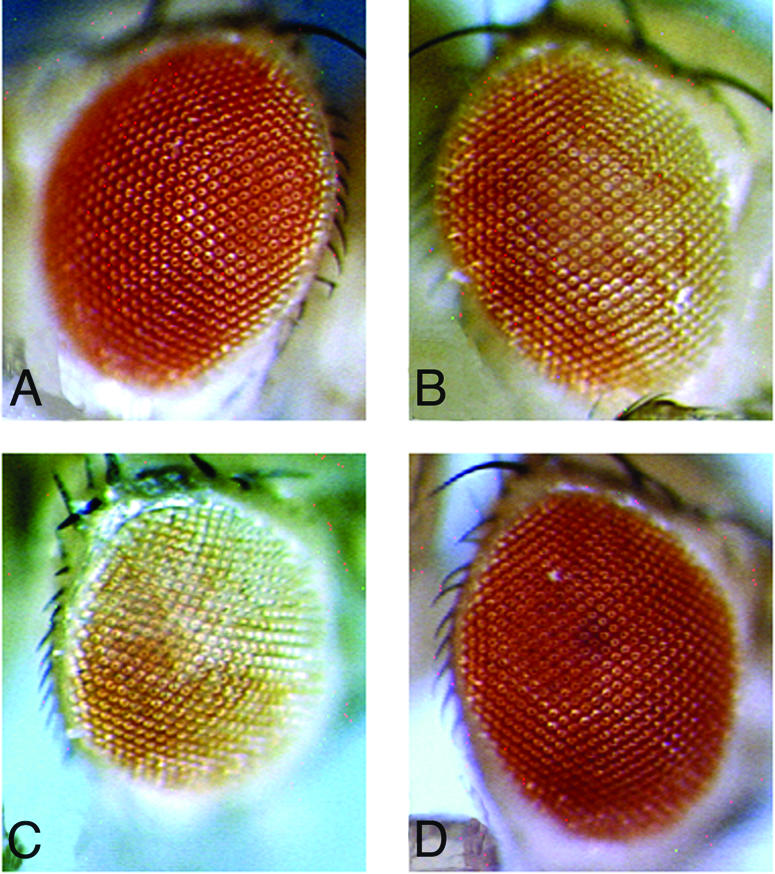
Fig. 1. GAL4–HP1 promotes variegation of the Wink-A7 transgenic line. (A) Eye of a Wink-A7 control male. (B) Heat shock induction of one GAL4–HP1 transgene results in variegation. (C) Heat shock induction of two GAL4–HP1 transgenes induces stronger variegation than induction of one. (D) Induction of GAL4–HP1 does not promote silencing after excision of GAL4 binding sites.
Fig. 2. Variegation of Wink-A7 augments with dose of GAL4–HP1. Eye pigment measurements on an average of 30 heads of adult flies. Lane 1, control males at 25°C; lane 2, control females at 25°C; lane 3, moderate (30°C) heat shock of males; lane 4, moderate (30°C) heat shock of females; lane 5, strong heat shock (37°C) of males; lane 6, strong heat shock (37°C) of females.
Fig. 3. Variegation of Wink-A7 responds to modifiers. Eye pigment measurements. Lane 1, Wink-A7 control male; lane 2, variegation caused by heat shock induction of GAL4–HP1; lane 3, partial suppression of variegation by a null mutation of Su(var)2-5 (Eissenberg et al., 1992); lane 4, an excess dose of HP1 does not enhance variegation of Wink-A7; the excess HP1 results from the heat-shock-inducible transgene in HSHP1.83C (Eissenberg et al., 1992); lane 5, partial suppression of variegation by the Su(var)3-7 mutation Df(3R)AceHD1; lane 6, an excess dose of Su(var)3-7 does not enhance variegation of Wink-A7; the excess Su(var)3-7 results from the heat-shock-inducible transgene HSFLTX (Cléard et al., 1995). Lanes 7 (control) and 8 (heat shock induction of GAL4–HP1), test of another insertion of Winkelried in euchromatin (Wink-S located at 43A). Variegation is not induced.
Improved accessibility to GAL4–HP1, or proximity to a heat shock locus, does not cause the specific variegated silencing of Wink-A7
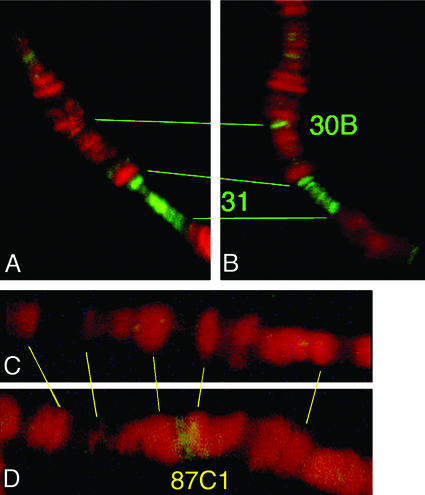
Why does the Wink-A7 insertion, and not the other five tested, respond to GAL4–HP1? A first possibility could be a difference in accessibility and binding of the transgene by the GAL4–HP1 fusion protein. This was examined by immunostaining of polytene chromosomes with an anti-GAL4 DNA-binding domain antibody. In the presence of GAL4–HP1, staining of the site of insertion of the transgene was seen in all strains, showing that the fusion protein recognizes the GAL4 binding sites in both non-variegating and variegating lines (see examples in Figure 4). Another difference among lines is the context of the site of insertion. We have mapped the insertion site of Wink-A7 at 87C (not shown, but see Figure 5 and DNA sequencing evidence reported below). This site coincides with the hsp70 heat shock gene locus at 87C1. Close proximity to a heat shock locus could result in a more open chromatin structure after heat shock induction of GAL4–HP1, and thus in a more efficient binding of the fusion protein. Having observed that the yeast GAL4 protein can function as a transcriptional activator in flies (not shown), we have tested this action on the Winkelried miniwhite gene of five of the six lines (including Wink-A7). The lines were combined with a transgenic strain producing the complete yeast GAL4 protein under the hsp70 heat shock promoter. In all cases, we see a similar increase of miniwhite expression due to binding of the GAL4 protein. Quantitatively, in the same experimental conditions as in Figure 2, the increase in optical density in Wink-A7 is 0.24 ± 0.01, while in the five other lines it ranges from 0.22 ± 0.01 to 0.26 ± 0.01. In summary, insertion of Winkelried in proximity to a heat shock locus seems not to affect Wink-A7 specifically for transcription activation compared with the other lines.
Fig. 4. The GAL4–HP1 fusion protein associates with the site of insertion of the reporter transgene. Immunofluorescent staining of polytene chromosomes with an antibody recognizing the DNA-binding domain of yeast GAL4. Green, fluorescein isothiocyanate-labelled anti-GAL4 antibody; red, propidium iodide staining of DNA. (A) Heterozygous Wink-A7 control. Staining at 31, a euchromatin site previously described as a binding site of HP1 (James et al., 1989), but not at 30B. (B) Heterozygous Wink-D. Staining at the site of insertion (one homologue at 30B) and at 31. (C) Heterozygous Wink-D control. No staining at 87C. (D) Heterozygous Wink-A7. Staining at the site of insertion in 87C.
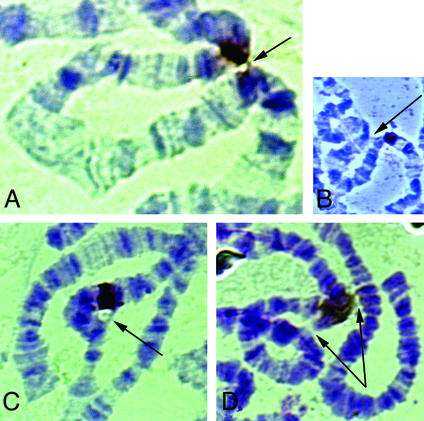
Fig. 5. Ectopic pairing of the Wink-A7 transgene promoted by GAL4–HP1. In situ hybridizations using the digoxygenin (DIG)-labelled plasmid pWH (Lu et al., 1996) as a probe. (A) Loop between 87C1 and 84D. (B) Loop between 87C1 and 89E (Bithorax Complex). (C) Loop between 87C1 and 86D. (D) Loop between 87C1, 84D and 94A. The sites associating with 87C were all previously described as intercalary heterochromatin (Zhimulev et al., 1982).
GAL4–HP1 induces loops between the Wink-A7 insertion site and intercalary, pericentric or telomeric heterochromatin in polytene chromosomes
We have examined the polytene chromosomes of the variegating Wink-A7 line after induction of GAL4–HP1 expression. In these chromosomes, the site of insertion of Winkelried at 87C is revealed by in situ hybridization with transgene sequences. To our surprise, in ∼26% (N = 366) of the chromosomes we observed the formation of loops extending from the insertion point in the Wink-A7 line (87C) to six sites on the right arm of the third chromosome (Figure 5). The loops connect 87C with 83D, 84D, 86D, 89E, 92D and 94A. Each one of these sites was described previously as one of the ∼10 intercalary heterochromatin domains reported by Zhimulev et al. (1982) on this chromosome arm, but neither 87C nor any of these sites of intercalary heterochromatin is known to be an HP1 binding site (James et al., 1989; our own unpublished results). Rarely, looping back of 87C was also observed with the chromocentre, the telomeres and other sites of 3R and 3L, and also with other chromosome arms (not shown). At low levels of GAL4–HP1 (no heat shock, stocks maintained at 18°C), only 7.3% (N = 165) of chromosomes exhibit loops. As a control, we have verified that the heat shock itself is not responsible for loop formation by observing chromosomes of the homozygous Wink-A7 line in the same temperature conditions. At 37°C, 6.9% (N = 86) of the chromosomes form loops, while 3.9% (N = 126) do so at 18°C. We conclude from these observations that targeting of HP1 to Winkelried at 87C results in association of this site with domains of intercalary, telomeric and pericentric heterochromatin. As already recounted, we have expressed a wild-type HP1 cloned under the hsp70 promoter. Heat shock of the homozygous transgene does not induce loops at 37°C at a higher level than the non-heat shock control (4.8%; N = 62). From these data, we conclude that the loops observed depend on the presence and binding of GAL4–HP1 at the ectopic site 87C.
GAL4–HP1 does not induce loops between the Wink-D insertion site and intercalary, pericentric or telomeric heterochromatin in polytene chromosomes
We have shown above that GAL4–HP1 promotes chromosome loops between its insertion site at 87C and sites of intercalary heterochromatin. Do the non-variegating lines also promote loops from their insertion site when GAL4–HP1 is induced? We have tested this hypothesis with the Wink-D line, a non-variegating line where Winkelried has landed at 30B on the second chromosome (Seum et al., 2000; Figure 4). In the presence of heat-shock-induced GAL4–HP1, we have observed loops originating from 30B in 6.5% (N = 573) of the chromosomes. In the same conditions, but in the absence of heat-shock-inducible GAL4–HP1, we have observed loops originating from 30B in 5% (N = 300) of the chromosomes. The frequencies are similar to those found for Wink-A7 in the absence of GAL4–HP1. We conclude that GAL4–HP1 does not promote ectopic chromosomal loops in the non-variegating line Wink-A7. Taken together, the results with Wink-A7 and Wink-D argue for a correlation between variegated silencing and ectopic pairing.
The transgene in Wink-A7 lies in middle repetitive sequences
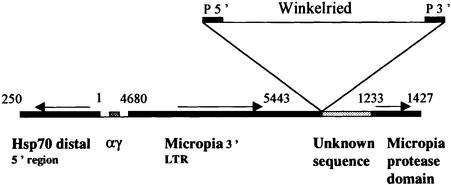
We still have to explain the specific capacity of variegated silencing of Wink-A7 compared with the other transgenic lines. To determine the sequence surrounding the insertion site in Wink-A7, we performed inverse PCR and sequenced the products. The transgene is inserted into a micropia retrotransposon (Lankenau and Hennig, 1990), next to αγ middle repetitive elements on one side, and next to the 5′ region of the distal hsp70 gene in 87C1 on the other side (Figure 6). By in situ hybridization of a micropia probe, we have determined that this element also maps at 84D, 69A, 42B, and at the centromere (not shown). 84D and 42B are described as sites of intercalary heterochromatin (Zhimulev et al., 1982). The αβ and αγ sequences have been identified next to the hsp70 locus by Lis and Hogness (1978). The 87C locus contains at least 12 tandemly repeated αβ and six αγ units, but the bulk of these units are located in centromeric heterochromatin. αγ sequences are also found at 42B, the localization of another micropia and of an intercalary heterochromatin site. Embedding of the reporter Winkelried in middle repetitive elements might play a role in this GAL4–HP1-dependent variegated silencing.
Fig. 6. Genomic sequence surrounding the Wink-A7 insertion site. The Winkelried transgene is inserted into a micropia retrotransposon (Lankenau and Hennig, 1990) and also next to a fragment of an αγ sequence (Lis and Hogness, 1978) and the 5′ region of the distal hsp70 gene (Ingolia et al., 1980; Karch et al., 1981). Base pair numbering refers to the authors cited.
GAL4–HP1 induces trans-inactivation of the homologue in Wink-A7
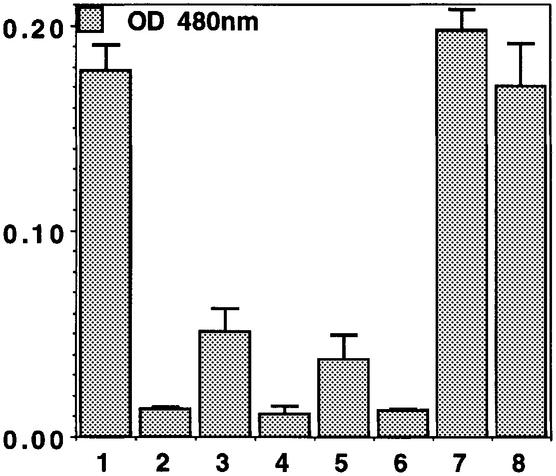
The results presented above suggest that HP1 promotes the association of Winkelried in Wink-A7 with intercalary, and more rarely with telomeric or pericentric heterochromatin. This association parallels variegated silencing. The next question is whether the tethering of HP1 to Wink-A7 on one chromosome can cause variegated silencing of a Wink-A7 reporter unable to bind GAL4–HP1 on the homologue. This phenomenon, trans-inactivation, has been detected for the variegation induced by a large insertion of heterochromatin near euchromatin genes (Henikoff and Dreesen, 1989). We have constructed a heterozygous line with a complete copy of Wink-A7 on one homologue, and a copy devoid of the binding sites for GAL4 on the other. This was made possible by the FRT sequences flanking the GAL4 binding sites in Winkelried (Seum et al., 2000). When the GAL4–HP1 transgene was combined with this line and induced by strong heat shocks, we did indeed observe trans-inactivation (Figure 7). The silencing is weaker than in cis, but is strictly dependent on GAL4–HP1 expression, and is not seen in the absence of the GAL4 binding sites. This suggests pairing of the homologues and assembly of an expanding repressive complex.

Fig. 7. Trans-inactivation of Winkelried by GAL4–HP1. (A) Wink-A7 control male. Eye pigment: 0.179 ± 0.005. (B) Wink-A7 variegation after induction of GAL4–HP1 in strong heat shock conditions. Eye pigment: 0.013 ± 0.007. (C) No variegation in Wink-A7 with GAL4 binding sites over Wink-A7 without GAL4 binding sites. Eye pigment: 0.324 ± 0.029. (D) Wink-A7 with GAL4 binding sites trans-inactivates Wink-A7 without GAL4 binding sites after heat shock induction of GAL4–HP1. Eye pigment: 0.055 ± 0.006. (E) Wink-A7 without GAL4 binding sites does not variegate. Eye pigment: 0.208 ± 0.004. (F) Induction of GAL4–HP1 does not induce variegation of Wink-A7 without GAL4 binding sites. Eye pigment: 0.167 ± 0.022.
Discussion
The mechanisms leading to position-effect variegation have been debated since its discovery by Müller (1930), and the finding by Schultz (1936) that it results from chromosomal rearrangements relocating the gene tested next to a block of pericentric heterochromatin. Polarity of euchromatic gene inactivation has supported models of linear expansion of the repressive state from the heterochromatin border [see Spofford (1976) for a review of genetic evidence]. Other observations have led to a spreading model where constituents of heterochromatin assemble from an existing block (Zuckerkandl, 1974; Tartof et al., 1984; Locke et al., 1988). Molecular studies have then shown that predictions concerning these constituents of heterochromatin are indeed fulfilled (i.e.Cléard et al., 1997). Nonetheless, if expansion of heterochromatin is a cause of variegation, the concept of linear spreading is in conflict with a number of observations. Expansion can ‘jump’ over genes (Talbert and Henikoff, 2000 and references therein) and examples of discontinuous expansion are also visible on polytene chromosomes (Belyaeva and Zhimulev, 1991). Whether expansion is strictly linear or discontinuous does not disqualify the spreading model, but examples of silencing at long distances cited below underlie a different mechanism. The brownD variegating rearrangement results from a megabase-sized insertion of satellite DNA causing folding back of the chromosome to pericentric heterochromatin (Csink and Henikoff, 1996; Dernburg et al., 1996). More striking, tandem arrays of middle repetitive elements can cause variegation of nearby genes (Dorer and Henikoff, 1994). This supports a pairing/looping model allowing for coalescence of dispersed repetitive elements among themselves and with pericentric heterochromatin, possibly through heterochromatin-associated proteins (discussed in Talbert and Henikoff, 2000). This coalescence could be facilitated by physical proximity of large blocks of heterochromatin, possibly by the resulting higher local concentration of the modifiers of variegation associated with heterochromatin.
Looping, as seen here, and the presumed higher availability of complex-forming proteins at blocks of heterochromatin explain both long-distance effects and expansion of repression observed in variegating rearrangements. Our experiments directly test and visualize some of these predictions. First, we find that variegation of a euchromatic insertion of a transgene seems to require two conditions: proximity of middle repetitive DNA, and local presence of HP1, a heterochromatin-associated protein and modifier of position-effect variegation. Indeed, overexpression of wild-type HP1 does not promote variegated silencing at 87C in the absence of HP1 at the site. HP1 must be targeted there by the GAL4 DNA-binding domain. Secondly, we observe that the region forms loops with sites of intercalary heterochromatin and with telomeric and pericentric heterochromatin. In contrast, in Wink-D, a non-variegating line, induction of GAL4–HP1 does not promote loops. Pairing and silencing appear correlated. Thirdly, HP1 targeting on one homologue trans-inactivates the reporter on the other. The chromosome pairing and looping promoted by HP1 result in trans-inactivation and variegation of a transgene. These observations place HP1 at a pivotal role. It interacts with Su(var)3-7 and recruits it at ectopic sites (Delattre et al., 2000). Su(var)3-7 is itself a protein found to interact with repetitive DNAs (F.Cléard and P.Spierer, submitted). Targeted HP1 may induce the pairing observed with domains of intercalary heterochromatin by recruiting Su(var)3-7 bound to middle repetitive elements near its 87C insertion site and at sites of intercalary, telomeric or pericentric heterochromatin. In a general model, position-effect variegation could result from expansion of heterochromatin blocks, but could also develop discontinuously by the attachment of Su(var)3-7, HP1 and other partners at dispersed middle repetitive sequences. The visible consequence is ectopic pairing within and among chromosomes.
We also speculate that genes in proximity to the anchoring sites of loops variegate when GAL4–HP1 is expressed. We have observed this for the miniwhite reporter of Wink-A7 and for its trans-inactivation on the homologous chromosome, but other genes should be tested, whether at 87C or at the sites of intercalary heterochromatin. As an example, the bithorax complex of homeotic genes lies at 89E, a region of intercalary heterochromatin able to loop with Wink-A7 in the presence of GAL4–HP1. In preliminary experiments, we did not detect homeotic phenotypes in the presence of GAL4–HP1; however, this needs a more comprehensive examination.
Materials and methods
Induction of GAL4–HP1
Heat shocks were carried out either constantly at 30°C during the whole development or according to the following protocol. Flies were allowed to lay at 25°C for 24 h. Embryos were then incubated at 30°C until the beginning of the pupal stage and then put into a water bath for three 37°C heat shocks per day until they reached adulthood.
Eye pigment measurements
Measurements were made according to Reuter and Wolff (1981).
Polytene chromosomes
For in situ hybridization of DNA probes, polytene chromosome squashes and hybridizations were performed as in Spierer et al. (1983), except that the probe was labelled with digoxygenin (DIG) and detected according to the DIG-DNA Labeling Kit protocol (Roche). For immunostaining, polytene chromosome squashes and staining were performed as described in Seum et al. (2000).
Inverse PCR
Inverse PCR was performed as in Cryderman (1998) with the following primers: GGTAAGCTTCGGCTATCGACGGGACCACC (P5′ out) and CGTGACTGTGCGTTAGGTCCTGTTCATTG (P5′ in) in the 5′ inverted repeat of the P element, and ATGTCTCTTGCCGACGGGACCACCTTATG (P3′ out) and GTTGATTAACCCTTAGCCCGTGGGG (P3′ in) in the 3′ inverted repeat of the P element. The enzymes used for restriction were BamHI and BglII in 3′ generating a 1.0 kb fragment, and Sau3A in 5′ generating a 1.7 kb fragment.
Acknowledgments
Acknowledgements
We thank Joël Eissenberg for the HSHP1.83C stock and Fabienne Cléard for the HSFLTX stock. This work was supported by grants from the Swiss National Science Foundation and by the State of Geneva.
REFERENCES
- Aagaard L. et al. (1999) Functional mammalian homologues of the Drosophila PEV-modifier Su(var)3-9 encode centromere-associated proteins which complex with the heterochromatin component M31. EMBO J., 18, 1923–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasland R. and Stewart,A.F. (1995) The chromo shadow domain, a second chromo domain in heterochromatin-binding protein 1, HP1. Nucleic Acids Res., 23, 3168–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsztein A.M., Kandels-Lewis,S.E., Mackay,A.M. and Earnshaw,W.C. (1998) INCENP centromere and spindle targeting: identification of essential conserved motifs and involvement of heterochromatin protein HP1. J. Cell Biol., 143, 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva E.S. and Zhimulev,I.F. (1991) Cytogenetic and molecular aspects of position effect variegation in Drosophila. III. Continuous and discontinuous compaction of chromosomal material as a result of position effect variegation. Chromosoma, 100, 453–466. [DOI] [PubMed] [Google Scholar]
- Cléard F., Matsarskaia,M. and Spierer,P. (1995) The modifier of position-effect variegation Suvar(3)7 of Drosophila: there are two alternative transcripts and seven scattered zinc fingers, each preceded by a tryptophan box. Nucleic Acids Res., 23, 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cléard F., Delattre,M. and Spierer,P. (1997) SU(VAR)3-7, a Drosophila heterochromatin-associated protein and companion of HP1 in the genomic silencing of position-effect variegation. EMBO J., 16, 5280–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryderman D.E., Cuaycong,M.H., Elgin,S.C. and Wallrath,L.L. (1998) Characterization of sequences associated with position-effect variegation at pericentric sites in Drosophila heterochromatin. Chromosoma, 107, 277–285. [DOI] [PubMed] [Google Scholar]
- Csink A.K. and Henikoff,S. (1996) Genetic modification of heterochromatic association and nuclear organization in Drosophila. Nature, 381, 529–531. [DOI] [PubMed] [Google Scholar]
- Delattre M., Spierer,A., Tonka,C. and Spierer,P. (2000) The genomic silencing of position-effect variegation in Drosophila melanogaster: interaction between the heterochromatin-associated protein Su(var)3-7 and HP1. J. Cell Sci., 113, 4253–4261. [DOI] [PubMed] [Google Scholar]
- Dernburg A.F., Broman,K.W., Fung,J.C., Marshall,W.F., Philips,J., Agard,D.A. and Sedat,J.W. (1996) Perturbation of nuclear architecture by long-distance chromosome interactions. Cell, 85, 745–759. [DOI] [PubMed] [Google Scholar]
- Dorer D.R. and Henikoff,S. (1994) Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell, 77, 993–1002. [DOI] [PubMed] [Google Scholar]
- Eissenberg J.C. and Elgin,S.C. (2000) The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev., 10, 204–210. [DOI] [PubMed] [Google Scholar]
- Eissenberg J.C., James,T.C., Foster-Hartnett,D.M., Hartnett,T., Ngan,V. and Elgin,S.C. (1990) Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl Acad. Sci. USA, 87, 9923–9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J.C., Morris,G.D., Reuter,G. and Hartnett,T. (1992) The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics, 131, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti L., Giovinazzo,G., Berloco,M. and Pimpinelli,S. (1998a) The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell, 2, 527–538. [DOI] [PubMed] [Google Scholar]
- Fanti L., Dorer,D.L., Berloco,M., Henikoff,S. and Pimpinelli,S. (1998b) Heterochromatin protein 1 binds transgene arrays. Chromosoma, 107, 286–292. [DOI] [PubMed] [Google Scholar]
- Frankel S., Sigel,E.A., Craig,C., Elgin,S.C., Mooseker,M.S. and Artavanis-Tsakonas,S. (1997) An actin-related protein in Drosophila colocalizes with heterochromatin protein 1 in pericentric heterochromatin. J. Cell Sci., 110, 1999–2012. [DOI] [PubMed] [Google Scholar]
- Henikoff S. and Dreesen,T.D. (1989) Trans-inactivation of the Drosophila brown gene: evidence for transcriptional repression and somatic pairing dependence. Proc. Natl Acad. Sci. USA, 86, 6704–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T.D., Craig,E.A. and McCarthy,B.J. (1980) Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell, 21, 669–679. [DOI] [PubMed] [Google Scholar]
- James T.C., Eissenberg,J.C., Craig,C., Dietrich,V., Hobson,A. and Elgin,S.C. (1989) Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur. J. Cell Biol., 50, 170–180. [PubMed] [Google Scholar]
- Karch F., Torok,I. and Tissières,A. (1981) Extensive regions of homology in front of the two hsp70 heat shock variant genes in Drosophila melanogaster. J. Mol. Biol., 148, 219–230. [DOI] [PubMed] [Google Scholar]
- Kellum R., Raff,J.W. and Alberts,B.M. (1995) Heterochromatin protein 1 distribution during development and during the cell cycle in Drosophila embryos. J. Cell Sci., 108, 1407–1418. [DOI] [PubMed] [Google Scholar]
- Koonin E.V., Zhou,S. and Lucchesi,J.C. (1995) The chromo superfamily: new members, duplication of the chromo domain and possible role in delivering transcription regulators to chromatin. Nucleic Acids Res., 23, 4229–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau D.H. and Hennig,W. (1990) Micropia-Dm2, the nucleotide sequence of a rearranged retrotransposon from Drosophila melanogaster. Nucleic Acids Res., 18, 4265–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin B., Nielsen,A.L., Garnier,J.M., Ichinose,H., Jeanmougin,F., Losson,R. and Chambon,P. (1996) A possible involvement of TIF1 α and TIF1 β in the epigenetic control of transcription by nuclear receptors. EMBO J., 15, 6701–6715. [PMC free article] [PubMed] [Google Scholar]
- Lehming N., Le Saux,A., Schuller,J. and Ptashne,M. (1998) Chromatin components as part of a putative transcriptional repressing complex. Proc. Natl Acad. Sci. USA, 95, 7322–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J.T. and Hogness,D.S. (1978) A novel arrangement of tandemly repeated genes at a major heat shock site in D.melanogaster. Cell, 14, 901–919. [DOI] [PubMed] [Google Scholar]
- Locke J., Kotarski,M.A. and Tartof,K.D. (1988) Dosage-dependent modifiers of position effect variegation in Drosophila and a mass action model that explains their effects. Genetics, 120, 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B.Y., Bishop,C.P. and Eissenberg,J.C. (1996) Developmental timing and tissue specificity of heterochromatin-mediated silencing. EMBO J., 15, 1323–1332. [PMC free article] [PubMed] [Google Scholar]
- Müller H.J. (1930) Types of viable variations induced by X-rays in Drosophila. J. Genet., 22, 299–334. [Google Scholar]
- Murzina N., Verreault,A., Laue,E. and Stillman,B. (1999) Hetero chromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell, 4, 529–540. [DOI] [PubMed] [Google Scholar]
- Pak D.T., Pflumm,M., Chesnokov,I., Huang,D.W., Kellum,R., Marr,J., Romanowski,P. and Botchan,M.R. (1997) Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell, 91, 311–323. [DOI] [PubMed] [Google Scholar]
- Reuter G. and Spierer,P. (1992) Position effect variegation and chromatin proteins. BioEssays, 14, 605–612. [DOI] [PubMed] [Google Scholar]
- Reuter G. and Wolff,I. (1981) Isolation of dominant suppressor mutations for position-effect variegation in Drosophila melanogaster. Mol. Gen. Genet., 182, 516–519. [DOI] [PubMed] [Google Scholar]
- Ryan R.F., Schultz,D.C., Ayyanathan,K., Singh,P.B., Friedman,J.R., Fredericks,W.J. and Rauscher,F.J.,III (1999) KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol. Cell. Biol., 19, 4366–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. (1936) Variegation in Drosophila and the inert chromosomal regions. Proc. Natl Acad. Sci. USA, 22, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler J.S., Marchio,A., Sitterlin,D., Transy,C. and Dejean,A. (1998) Interaction of SP100 with HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc. Natl Acad. Sci. USA, 95, 7316–7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seum C., Spierer,A., Delattre,M., Pauli,D. and Spierer,P. (2000) A GAL4–HP1 fusion protein targeted near heterochromatin promotes gene silencing. Chromosoma, 109, 453–459. [DOI] [PubMed] [Google Scholar]
- Spierer P., Spierer,A., Bender,W. and Hogness,D.S. (1983) Molecular mapping of genetic and chromomeric units in Drosophila melanogaster. J. Mol. Biol., 168, 35–50. [DOI] [PubMed] [Google Scholar]
- Spofford J.B. (1976) Position-effect variegation in Drosophila. In Ashburner,M. and Novitski,E. (eds), The Genetics and Biology of Drosophila. Vol. 1c. Academic Press, London, UK, pp. 595–1018.
- Talbert P.B. and Henikoff,S. (2000) A reexamination of spreading of position-effect variegation in the white-roughest region of Drosophila melanogaster. Genetics, 154, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof K.D., Hobbs,C. and Jones,M. (1984) A structural basis for variegating position effects. Cell, 37, 869–878. [DOI] [PubMed] [Google Scholar]
- Tschiersch B., Hofmann,A., Krauss,V., Dorn,R., Korge,G. and Reuter,G. (1994) The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3-9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J., 13, 3822–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrath L.L. (1998) Unfolding the mysteries of heterochromatin. Curr. Opin. Genet. Dev., 8, 147–153. [DOI] [PubMed] [Google Scholar]
- Ye Q. and Worman,H.J. (1996) Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J. Biol. Chem., 271, 14653–14656. [DOI] [PubMed] [Google Scholar]
- Ye Q., Callebaut,I., Pezhman,A., Courvalin,J.C. and Worman,H.J. (1997) Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBR. J. Biol. Chem., 272, 14983–14989. [DOI] [PubMed] [Google Scholar]
- Zhimulev I.F., Semeshin,V.F., Kulichkov,V.A. and Belyaeva,E.S. (1982) Intercalary heterochromatin in Drosophila. Chromosoma, 87, 197–228. [Google Scholar]
- Zuckerkandl E. (1974) A possible role of ‘inert’ heterochromatin in cell differentiation. Action of and competition for ‘locking’ molecules. Biochimie, 56, 937–954. [DOI] [PubMed] [Google Scholar]