Abstract
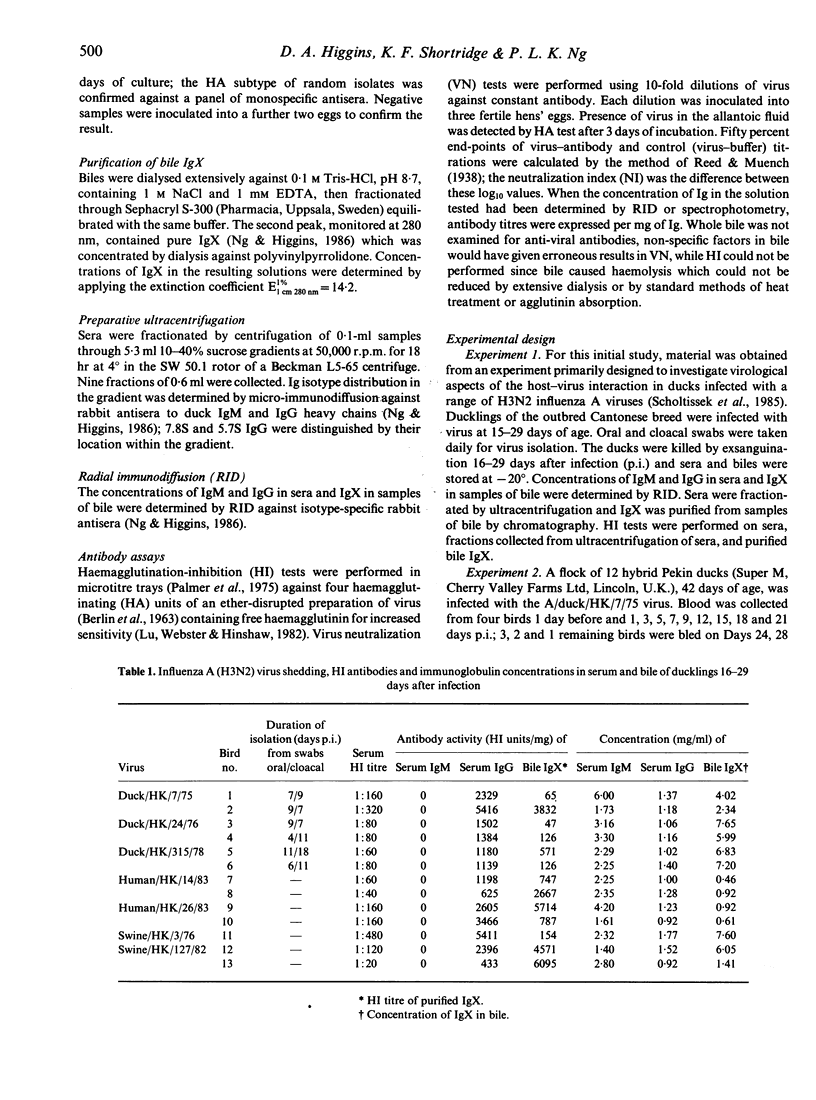
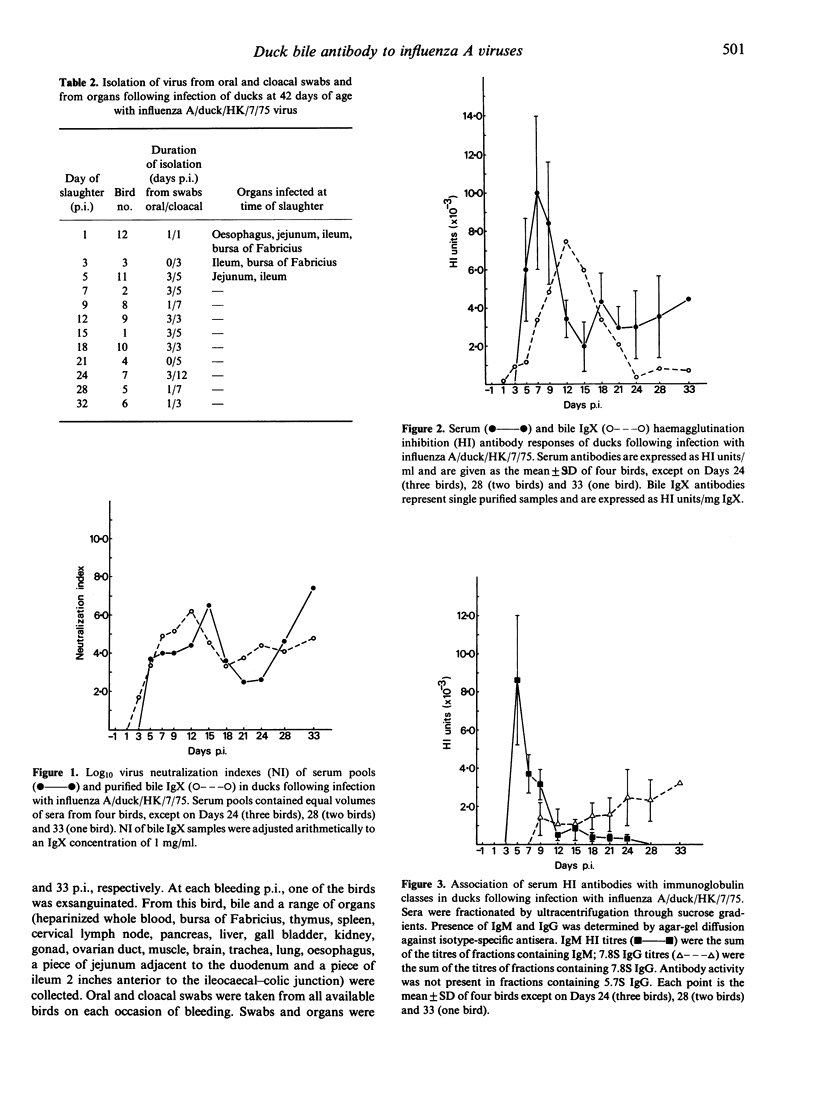
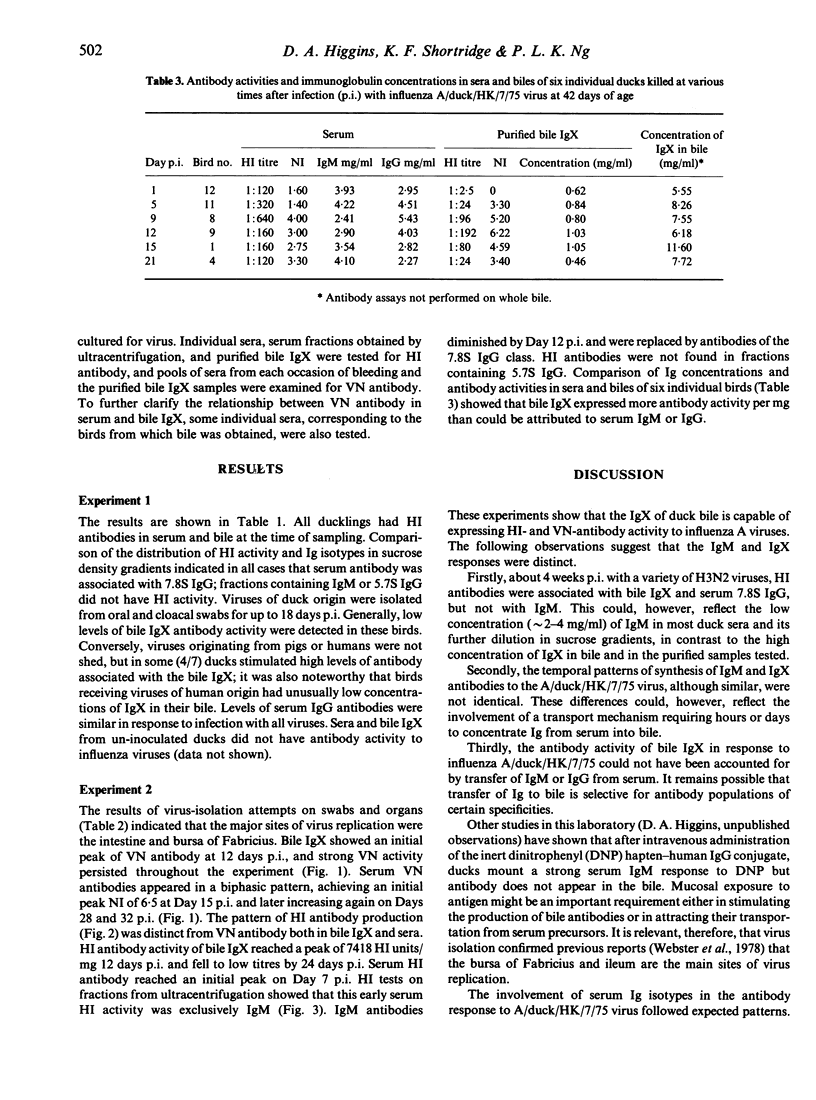
The capacity of the IgM-like bile immunoglobulin (IgX) of the duck (Anas platyrhynchos) to express antibody activity to H3N2 influenza A viruses, and the dependence of this activity on the co-existence of serum IgM antibodies were investigated. Ducklings infected orally and intranasally at 15-29 days of age with viruses isolated from different host species were examined for haemagglutination-inhibiting (HI) antibodies in biles and sera 16-29 days after infection (p.i.). All biles had antibodies associated with IgX; all sera had antibodies associated only with the 7.8S IgG. Following oral infection of birds 42-days-old with influenza A/duck/HK/7/75 virus, serum HI antibodies were an initial IgM response occurring from 5-12 days p.i., followed by the appearance of 7.8S IgG antibodies. Virus-neutralizing (VN) antibodies in serum were also biphasic; isotype classification was not attempted. Bile IgX developed HI and VN activity. HI antibodies reached peak titres 12 days p.i. and fell to low levels by 24 days p.i. VN antibodies also reached peak titres 12 days p.i., but thereafter persisted at quite high levels throughout the experiment. Development of high titres of antibody in bile coincided with the termination of virus excretion in faeces. These experiments confirm that bile IgX of the duck can function as antibody in response to influenza A viruses, and that its activity appears to be independent of serum IgM. Its possible relevance in determining survival of virus in the intestine is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLIN B. S., MCQUEEN J. L., MINUSE E., DAVENPORT F. M. A METHOD FOR INCREASING THE SENSITIVITY OF THE HEMAGGLUTINATION-INHIBITION TEST WITH EQUINE INFLUENZA VIRUS. Virology. 1963 Dec;21:665–666. doi: 10.1016/0042-6822(63)90244-7. [DOI] [PubMed] [Google Scholar]
- Grey H. M. Duck immunoglobulins. I. Structural studies on a 5.7S and 7.8S gamma-globulin. J Immunol. 1967 Apr;98(4):811–819. [PubMed] [Google Scholar]
- Grey H. M. Duck immunoglobulins. II. Biologic and immunochemical studies. J Immunol. 1967 Apr;98(4):820–826. [PubMed] [Google Scholar]
- Higgins D. A., Calnek B. W. Fowl immunoglobulins: quantitation and antibody activity during Marek's disease in genetically resistant and susceptible birds. Infect Immun. 1975 Jan;11(1):33–41. doi: 10.1128/iai.11.1.33-41.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E., Flajnik M. F., Du Pasquier L. A third immunoglobulin class in amphibians. J Immunol. 1985 Sep;135(3):1998–2004. [PubMed] [Google Scholar]
- Jackson G. D., Lemaître-Coelho I., Vaerman J. P., Bazin H., Beckers A. Rapid disappearance from serum of intravenously injected rat myeloma IgA and its secretion into bile. Eur J Immunol. 1978 Feb;8(2):123–126. doi: 10.1002/eji.1830080210. [DOI] [PubMed] [Google Scholar]
- Jackson G. D., Walker P. G., Schiff J. M., Barrington P. J., Fisher M. M., Underdown B. J. A role for the spleen in the appearance of IgM in the bile of rats injected intravenously with horse erythrocytes. J Immunol. 1985 Jul;135(1):152–157. [PubMed] [Google Scholar]
- Kida H., Yanagawa R., Matsuoka Y. Duck influenza lacking evidence of disease signs and immune response. Infect Immun. 1980 Nov;30(2):547–553. doi: 10.1128/iai.30.2.547-553.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Hanson R. P. Effects of bile and gastrointestinal secretions on the infectivity of Newcastle disease virus. Infect Immun. 1975 Apr;11(4):692–697. doi: 10.1128/iai.11.4.692-697.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb C. J., Clem L. W. Phylogeny of immunoglobulin structure and function-XII. Secretory immunoglobulins in the bile of the marine teleost Archosargus probatocephalus. Mol Immunol. 1981 Jul;18(7):615–619. doi: 10.1016/0161-5890(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Lu B. L., Webster R. G., Hinshaw V. S. Failure to detect hemagglutination-inhibiting antibodies with intact avian influenza virions. Infect Immun. 1982 Nov;38(2):530–535. doi: 10.1128/iai.38.2.530-535.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning R. J., Walker P. G., Carter L., Barrington P. J., Jackson G. D. Studies on the origins of biliary immunoglobulins in rats. Gastroenterology. 1984 Jul;87(1):173–179. [PubMed] [Google Scholar]
- Markwell D. D., Shortridge K. F. Possible waterborne transmission and maintenance of influenza viruses in domestic ducks. Appl Environ Microbiol. 1982 Jan;43(1):110–115. doi: 10.1128/aem.43.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett A. P., Rose M. E. Immune responses to eimeria: quantification of antibody isotypes to Eimeria tenella in chicken serum and bile by means of the ELISA. Parasite Immunol. 1986 Sep;8(5):481–489. doi: 10.1111/j.1365-3024.1986.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Shaw L. J., Fitzharris B., Peppard J., Hamilton M. J., Simpson M. T., Hunt T. M., Hinton R. H. Sources of proteins in human bile. Gut. 1985 May;26(5):500–509. doi: 10.1136/gut.26.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P. L., Higgins D. A. Bile immunoglobulin of the duck (Anas platyrhynchos). I. Preliminary characterization and ontogeny. Immunology. 1986 Jun;58(2):323–327. [PMC free article] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard J. V., Jackson L. E., Hall J. G. The occurrence of secretory IgM in the bile of rats. Clin Exp Immunol. 1983 Sep;53(3):623–626. [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Bürger H., Kistner O., Shortridge K. F. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985 Dec;147(2):287–294. doi: 10.1016/0042-6822(85)90131-x. [DOI] [PubMed] [Google Scholar]
- Shortridge K. F. Avian influenza A viruses of southern China and Hong Kong: ecological aspects and implications for man. Bull World Health Organ. 1982;60(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Shortridge K. F., King A. P., Webster R. G. Monoclonal antibodies for characterizing H3N2 influenza viruses that persist in pigs in China. J Infect Dis. 1987 Mar;155(3):577–581. doi: 10.1093/infdis/155.3.577. [DOI] [PubMed] [Google Scholar]
- Toth T. E., Norcross N. L. Immunoelectrophoresis of duck sera and immunoglobulins. Avian Dis. 1981 Jan-Mar;25(1):1–10. [PubMed] [Google Scholar]
- Vuitton D. A., Seilles E., Claude P., Sava P., Delacroix D. L. Gall bladder: the predominant source of bile IgA in man? Clin Exp Immunol. 1985 Oct;62(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Yakhno M., Hinshaw V. S., Bean W. J., Murti K. G. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology. 1978 Feb;84(2):268–278. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman B., Shalatin N., Grey H. M. Structural studies on the duck 5.7S and 7.8S immunoglobulins. Biochemistry. 1971 Feb 2;10(3):482–488. doi: 10.1021/bi00779a021. [DOI] [PubMed] [Google Scholar]


