Abstract
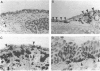
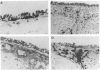
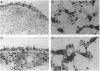
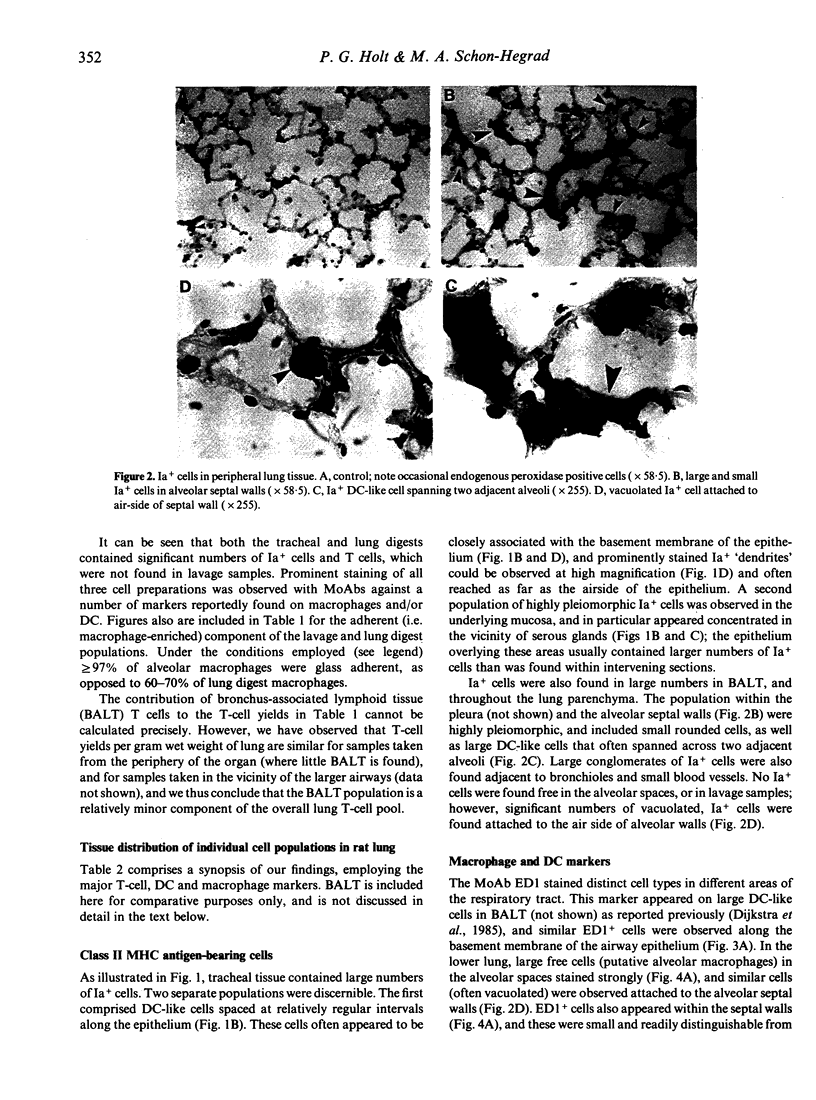
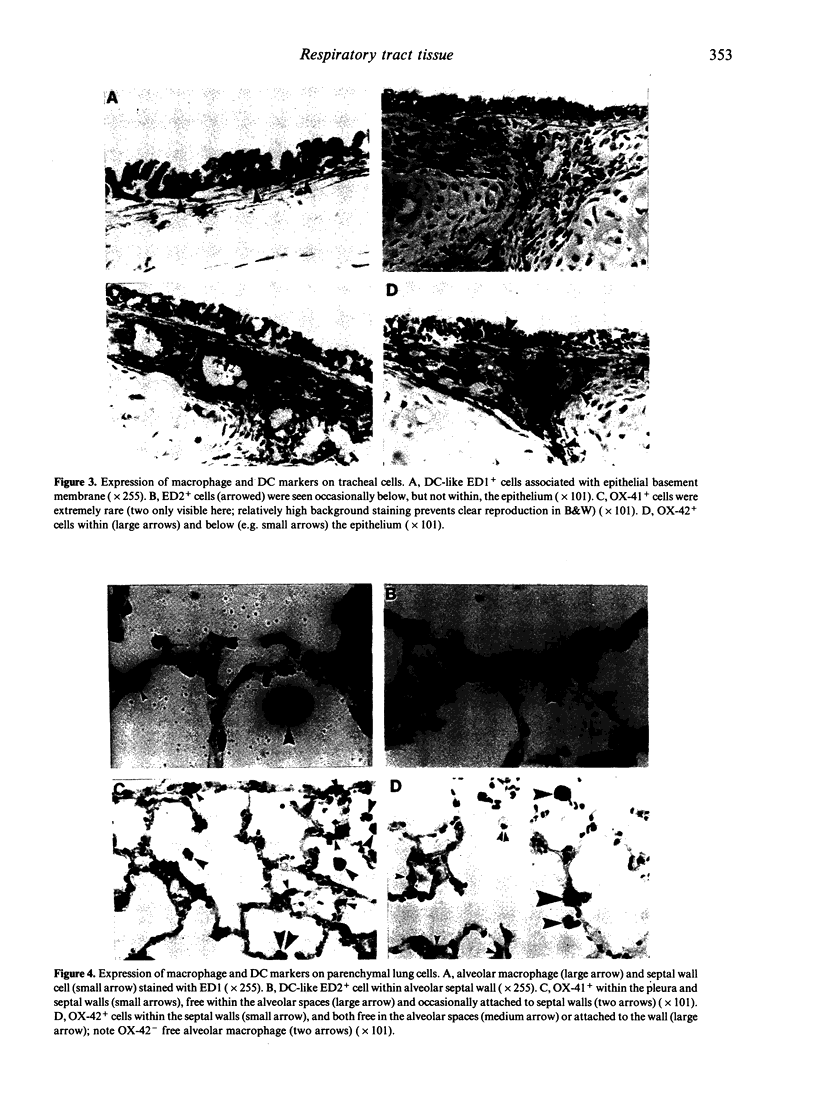
Monoclonal antibodies against a range of surface markers were used to localize T cells, macrophages and dendritic cells (DC) in sections of rat trachea and peripheral lung, employing the immunoperoxidase technique. A population of Ia-bearing cells with characteristic DC morphology was identified within the tracheal epithelium, closely associated with the basement membrane, and Ia+ dendritic processes from these cells penetrated the epithelium reaching the overlying fluid layer. A second DC-like population, also Ia+ but differing from the airway DC in expression of other markers, was identified within the alveolar septal walls. Both types of DC were intimately associated with populations of pleiomorphic heterogenously staining macrophages. In addition, a large population of T lymphocytes was identified within the alveolar septa; the lymphocytes occurred as single, isolated cells, distributed randomly throughout the lung parenchyma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. Thymic macrophages modulate one stage of T cell differentiation in vitro. J Immunol. 1978 Nov;121(5):1861–1864. [PubMed] [Google Scholar]
- Bursuker I., Goldman R. On the origin of macrophage heterogeneity: a hypothesis. J Reticuloendothel Soc. 1983 Mar;33(3):207–220. [PubMed] [Google Scholar]
- Campbell D. A., Poulter L. W., du Bois R. M. Immunocompetent cells in bronchoalveolar lavage reflect the cell populations in transbronchial biopsies in pulmonary sarcoidosis. Am Rev Respir Dis. 1985 Dec;132(6):1300–1306. doi: 10.1164/arrd.1985.132.6.1300. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Enander I., Ahlstedt S., Nygren H. Mononuclear cells, mast cells and mucous cells as part of the delayed hypersensitivity response to aerosolized antigen in mice. Immunology. 1984 Apr;51(4):661–668. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. I. A simple technique for the preparation of high numbers of viable alveolar macrophages from small laboratory animals. J Immunol Methods. 1979;27(2):189–198. doi: 10.1016/0022-1759(79)90264-3. [DOI] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. II. Inhibition of lymphocyte proliferation by purified macrophages from rat lung. Immunology. 1979 Jun;37(2):429–436. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., O'Leary C., Krska K., Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985 Dec;62(3):586–593. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., Venaille T., O'Leary C., Krska K., Flexman J., Farrell H., Shellam G., Young P., Penhale J. Preparation of interstitial lung cells by enzymatic digestion of tissue slices: preliminary characterization by morphology and performance in functional assays. Immunology. 1985 Jan;54(1):139–147. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986 Feb;63(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Robinson B. W., Reid M., Kees U. R., Warton A., Dawson V. H., Rose A., Schon-Hegrad M., Papadimitriou J. M. Extraction of immune and inflammatory cells from human lung parenchyma: evaluation of an enzymatic digestion procedure. Clin Exp Immunol. 1986 Oct;66(1):188–200. [PMC free article] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., Bowers W. E. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982 Jul 1;156(1):1–19. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., O'Brien J. P., Beyer C. F., Bowers W. E. Rat dendritic cells function as accessory cells and control the production of a soluble factor required for mitogenic responses of T lymphocytes. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5414–5418. doi: 10.1073/pnas.77.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Holt P. G., Papadimitriou J. M. Functional characteristics of the veiled cells in afferent lymph from the rat intestine. Immunology. 1986 Jul;58(3):379–387. [PMC free article] [PubMed] [Google Scholar]
- Pennline K. J., Herscowitz H. B. Dual role for alveolar macrophages in humoral and cell-mediated immune responses: evidence for suppressor and enhancing functions. J Reticuloendothel Soc. 1981 Sep;30(3):205–217. [PubMed] [Google Scholar]
- Pugh C. W., MacPherson G. G., Steer H. W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983 Jun 1;157(6):1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. P., Puklavec M., Mason D. W. MRC OX-52: a rat T-cell antigen. Immunology. 1986 Apr;57(4):527–531. [PMC free article] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. MRC OX-43: a monoclonal antibody which reacts with all vascular endothelium in the rat except that of brain capillaries. Immunology. 1986 Feb;57(2):231–237. [PMC free article] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Sertl K., Takemura T., Tschachler E., Ferrans V. J., Kaliner M. A., Shevach E. M. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986 Feb 1;163(2):436–451. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C. Heterogeneity of macrophages in response to lymphokines and other signals. Mol Immunol. 1982 Oct;19(10):1275–1278. doi: 10.1016/0161-5890(82)90293-0. [DOI] [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G. J., Dijkstra C. D., Sminia T. Characterization of pulmonary macrophages and bronchus-associated lymphoid tissue (BALT) macrophages in the rat. An enzyme-cytochemical and immunocytochemical study. Immunobiology. 1985 Jul;169(5):553–562. doi: 10.1016/S0171-2985(85)80009-7. [DOI] [PubMed] [Google Scholar]