Abstract
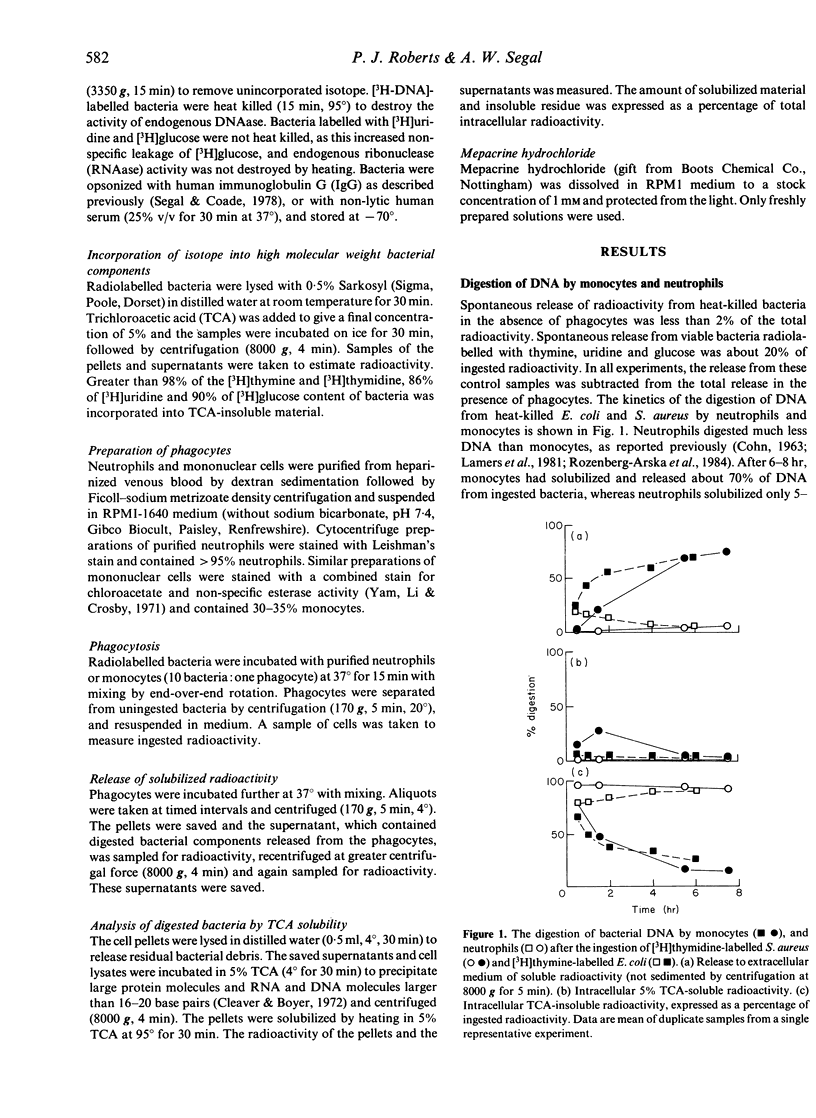
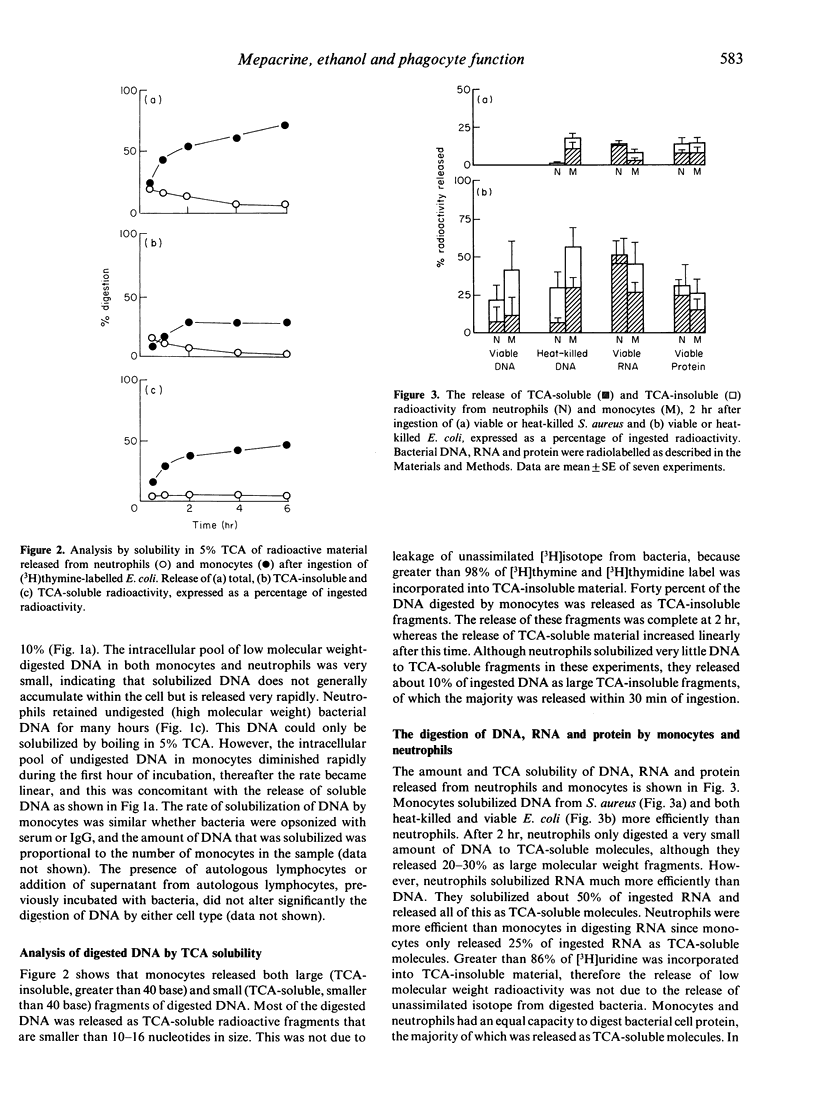
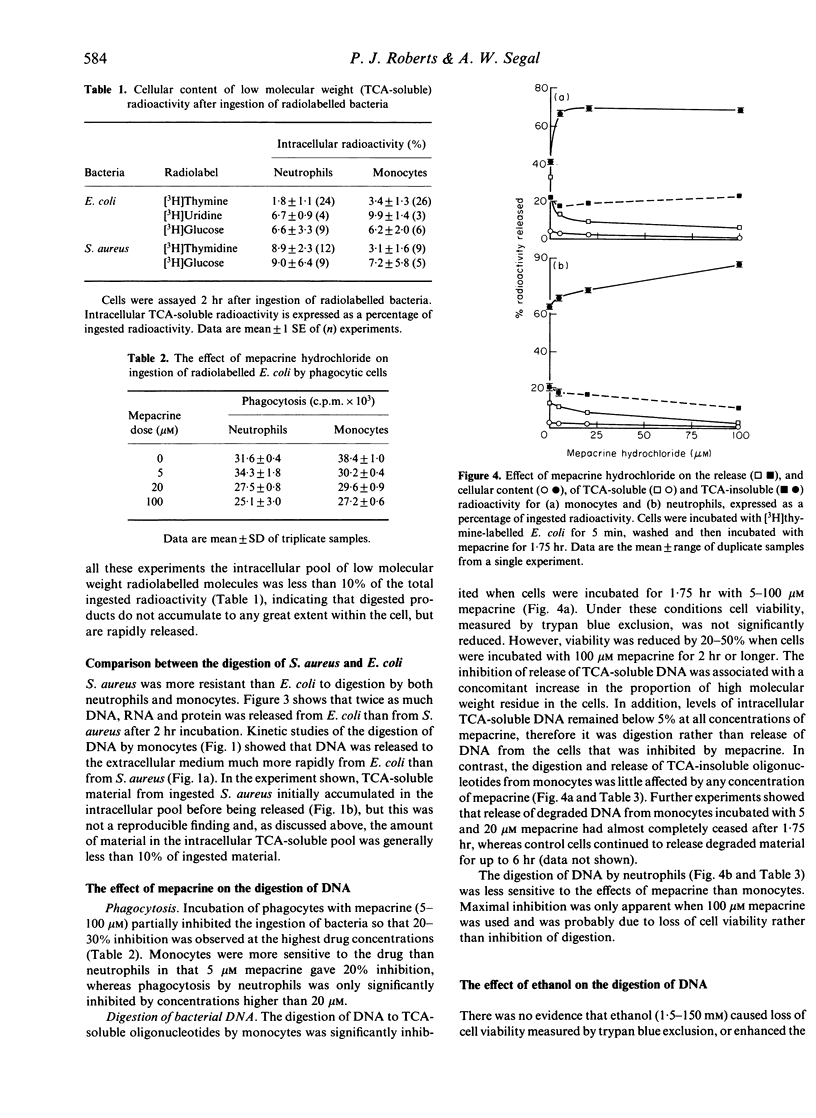
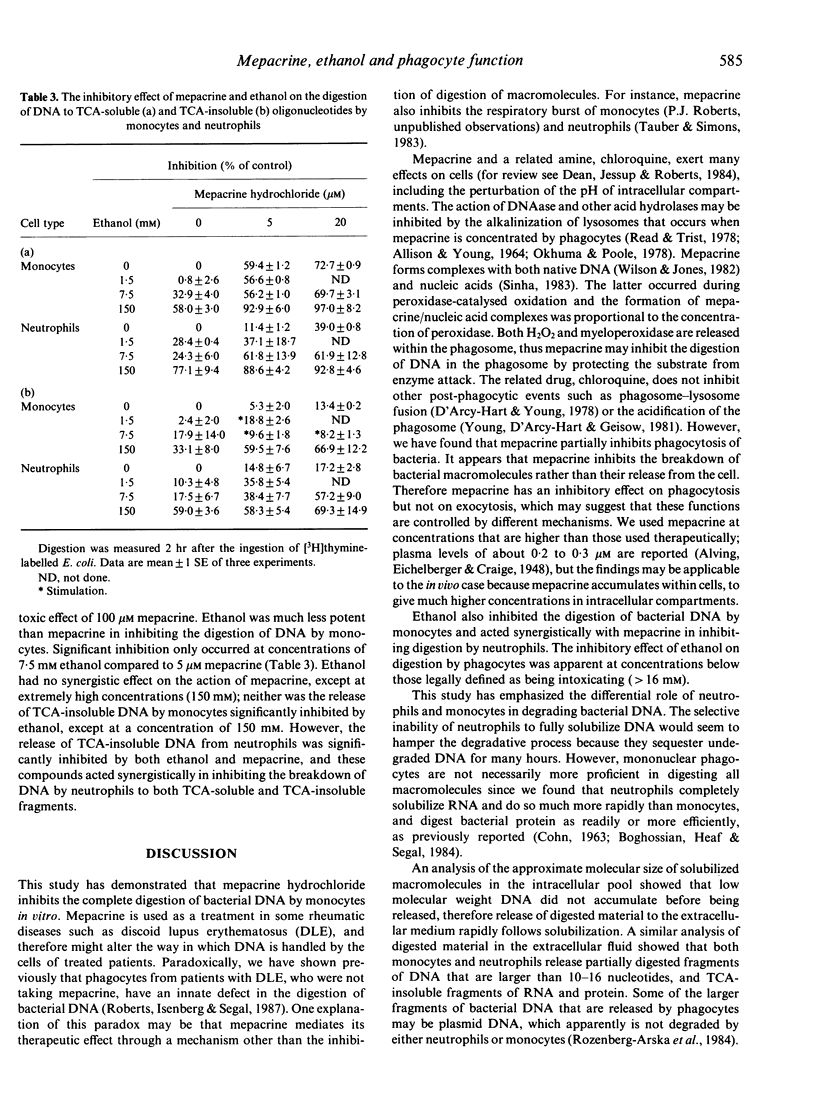
The digestion of radiolabelled DNA, RNA and protein from two species of bacteria was measured after their ingestion by neutrophils and monocytes. The Escherichia coli (W3110T-) strain was more susceptible to digestion than Staphylococcus aureus. Monocytes solubilized the majority of bacterial DNA to trichloroacetic acid (TCA)-soluble oligonucleotides, which were released rapidly from the cell, whereas neutrophils digested little DNA and retained most as a high molecular weight residue. Both monocytes and neutrophils released about 30% of DNA as large TCA-insoluble fragments. However, neutrophils were more proficient than monocytes at degrading bacterial RNA. Monocytes and neutrophils digested bacterial protein equally well. The drug mepacrine hydrochloride inhibited phagocytosis of bacteria by both neutrophils and monocytes, although monocytes were more sensitive to the drug. Mepacrine specifically inhibited the terminal digestion of DNA by monocytes to TCA-soluble molecules, whereas digestion to large molecular weight fragments apparently was not affected. Ethanol also inhibited the breakdown of DNA by phagocytic cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., YOUNG M. R. UPTAKE OF DYES AND DRUGS BY LIVING CELLS IN CULTURE. Life Sci. 1964 Dec;3:1407–1414. doi: 10.1016/0024-3205(64)90082-7. [DOI] [PubMed] [Google Scholar]
- Alving A. S., Eichelberger L., Craige B., Jones R., Whorton C. M., Pullman T. N. STUDIES ON THE CHRONIC TOXICITY OF CHLOROQUINE (SN-7618). J Clin Invest. 1948 May;27(3 Pt 2):60–65. doi: 10.1172/JCI101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton R. G., Stokes P. E., Schwartz M. S., Louria D. B. Effect of alcohol and various diseases on leukocyte mobilization, phagocytosis and intracellular bacterial killing. N Engl J Med. 1970 Jan 15;282(3):123–128. doi: 10.1056/NEJM197001152820303. [DOI] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Boyer H. W. Solubility and dialysis limits of DNA oligonucleotides. Biochim Biophys Acta. 1972 Mar 14;262(2):116–124. doi: 10.1016/0005-2787(72)90224-9. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Rowan-Kelly B., Seow W. K., Thong Y. H. Depression of human polymorphonuclear leucocyte function by anti-malarial drugs. Immunology. 1986 May;58(1):125–130. [PMC free article] [PubMed] [Google Scholar]
- Gluckman S. J., MacGregor R. R. Effect of acute alcohol intoxication on granulocyte mobilization and kinetics. Blood. 1978 Sep;52(3):551–559. [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Manipulations of the phagosome-lysosome fusion response in cultured macrophages. Enhancement of fusion by chloroquine and other amines. Exp Cell Res. 1978 Jul;114(2):486–490. doi: 10.1016/0014-4827(78)90516-5. [DOI] [PubMed] [Google Scholar]
- Lamers M. C., De Groot E. R., Roos D. Phagocytosis and degradation of DNA-anti-DNA complexes by human phagocytes. I. Assay conditions, quantitative aspects and differences between human blood monocytes and neutrophils. Eur J Immunol. 1981 Oct;11(10):757–764. doi: 10.1002/eji.1830111005. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Antioxidant action of antimalarials. Ann Rheum Dis. 1986 Mar;45(3):244–248. doi: 10.1136/ard.45.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. J., Isenberg D. A., Segal A. W. Defective degradation of bacterial DNA by phagocytes from patients with systemic and discoid lupus erythematosus. Clin Exp Immunol. 1987 Jul;69(1):68–78. [PMC free article] [PubMed] [Google Scholar]
- Rozenberg-Arska M., van Strijp J. A., Hoekstra W. P., Verhoef J. Effect of human polymorphonuclear and mononuclear leukocytes on chromosomal and plasmid DNA of Escherichia coli. Role of acid DNase. J Clin Invest. 1984 May;73(5):1254–1262. doi: 10.1172/JCI111327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Coade S. B. Kinetics of oxygen consumption by phagocytosing human neutrophils. Biochem Biophys Res Commun. 1978 Oct 16;84(3):611–617. doi: 10.1016/0006-291x(78)90749-0. [DOI] [PubMed] [Google Scholar]
- Sinha B. K. Irreversible binding of quinacrine to nucleic acids during horseradish peroxidase- and prostaglandin synthetase-catalyzed oxidation. Biochem Pharmacol. 1983 Sep 1;32(17):2604–2607. doi: 10.1016/0006-2952(83)90028-x. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Simons E. R. Dissociation of human neutrophil membrane depolarization, respiratory burst stimulation and phospholipid metabolism by quinacrine. FEBS Lett. 1983 May 30;156(1):161–164. doi: 10.1016/0014-5793(83)80269-5. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Jones R. L. Interaction of actinomycin D, ethidium, quinacrine, daunorubicin, and tetralysine with DNA: 31P NMR chemical shift and relaxation investigation. Nucleic Acids Res. 1982 Feb 25;10(4):1399–1410. doi: 10.1093/nar/10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Young M. R., Hart P. D., Geisow M. J. Action of weak bases on phagosomes of cultured macrophages. Suppression by ammonium ions of an early increase in phagosomal pH. Exp Cell Res. 1981 Oct;135(2):442–445. doi: 10.1016/0014-4827(81)90187-7. [DOI] [PubMed] [Google Scholar]


