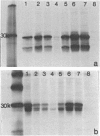
Abstract
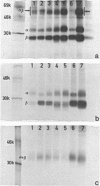
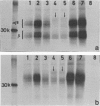
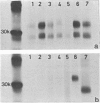
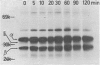
The physicochemical features, biosynthesis and glycosylation of sheep class II molecules were investigated using a panel of seven monoclonal antibodies. The class II molecules recognized by different monoclonal antibodies could be differentiated using SDS-PAGE. Two monoclonal antibodies, SBU.II 42-20 and 49-1, reacted with dissociated sheep class II molecules and recognized epitopes on class II alpha and beta polypeptides, respectively. The structure of the sheep class II heterodimer differed from that of mouse and man in that it was unstable in the presence of 1% SDS at 20 degrees and, following reduction, sheep beta polypeptides displayed a marked increase in MW, resulting in the apparent co-migration of reduced alpha and beta polypeptides on SDS-PAGE. This phenomenon was not seen using sheep class II molecules synthesized in the presence of tunicamycin. Pulse-chase analyses of biosynthetically labelled sheep class II molecules suggested the rapid association and glycosylation of sheep class II alpha and beta polypeptides during synthesis. Both alpha and beta polypeptides of sheep class II molecules carried N-linked oligosaccharides of MW 6,000 and 3,000, respectively. However, unlike human class II oligosaccharides, these were exclusively of the complex or sialylated type.
Full text
PDF

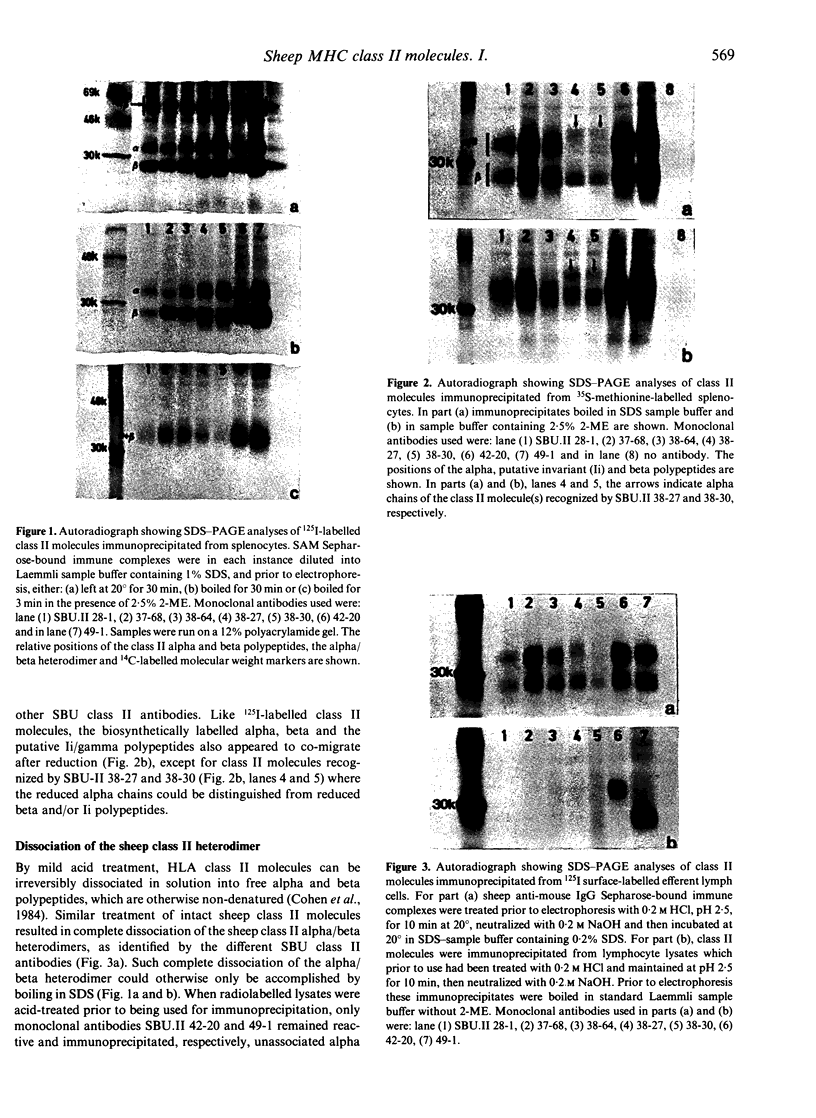
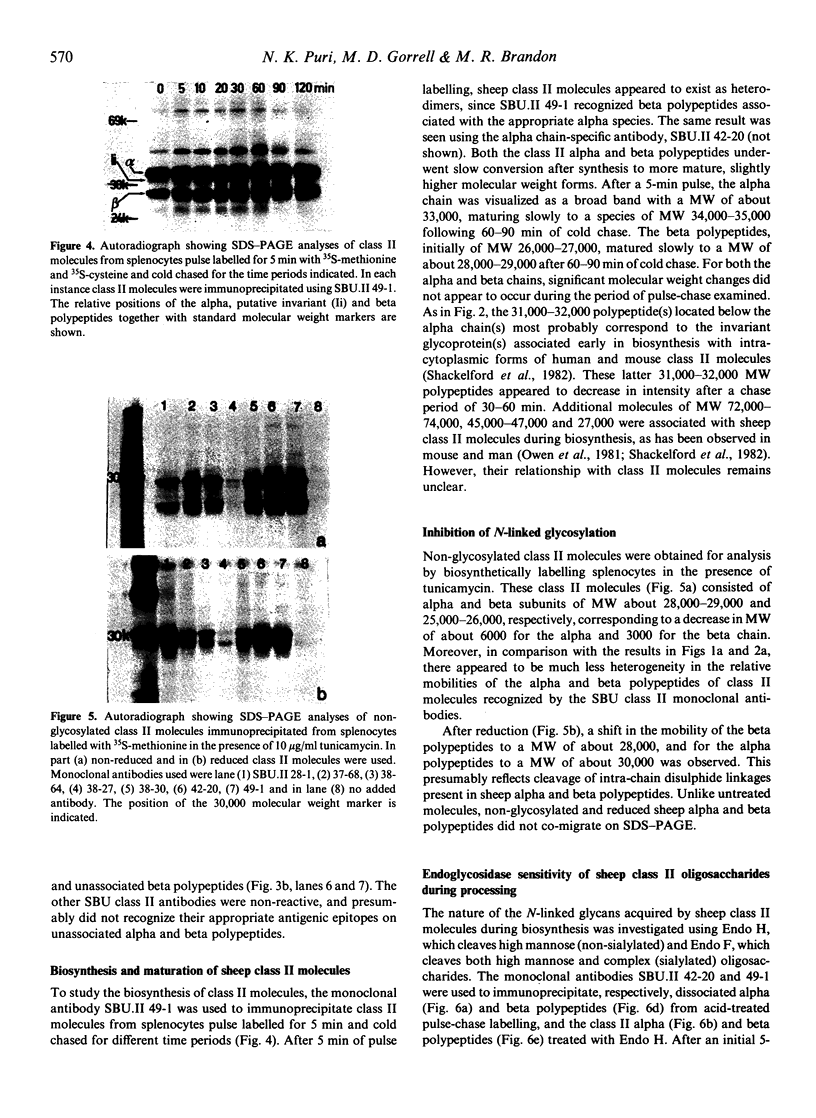
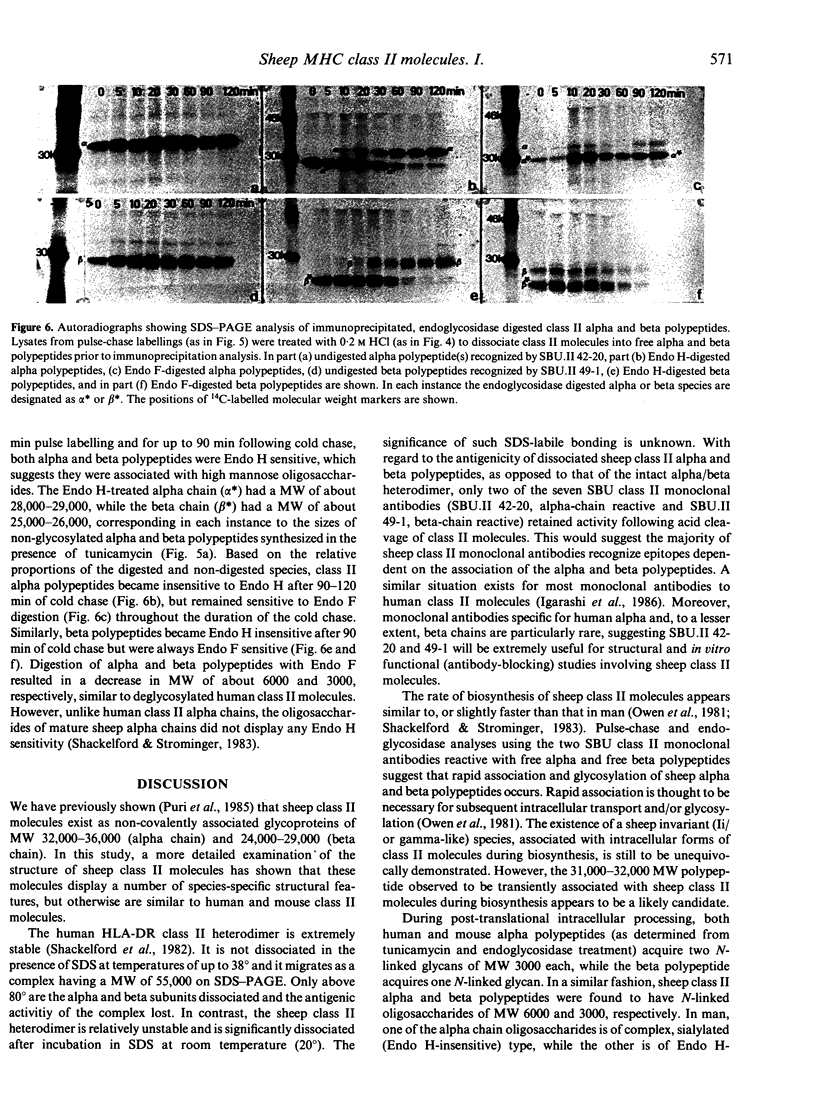


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen B. B., Moxley M., Crichton D., Deane D. L., Steel C. M. A mild procedure for separating polypeptide chains prior to immunoprecipitation and western blotting analysis. J Immunol Methods. 1984 Dec 14;75(1):99–105. doi: 10.1016/0022-1759(84)90229-1. [DOI] [PubMed] [Google Scholar]
- Cowan E. P., Cummings R. D., Lee D. R., Schwartz B. D., Cullen S. E. Structural characterization of murine Ia antigen N-linked oligosaccharides and localization of specific structures to two unique alpha-chain glycosylation sites. Mol Immunol. 1985 Feb;22(2):135–143. doi: 10.1016/s0161-5890(85)80007-9. [DOI] [PubMed] [Google Scholar]
- Giles R. C., Capra J. D. Structure, function, and genetics of human class II molecules. Adv Immunol. 1985;37:1–71. doi: 10.1016/s0065-2776(08)60337-5. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- HALL J. G., MORRIS B. The output of cells in lymph from the popliteal node of sheep. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:360–369. doi: 10.1113/expphysiol.1962.sp001620. [DOI] [PubMed] [Google Scholar]
- Hopkins J., Dutia B. M., McConnell I. Monoclonal antibodies to sheep lymphocytes. I. Identification of MHC class II molecules on lymphoid tissue and changes in the level of class II expression on lymph-borne cells following antigen stimulation in vivo. Immunology. 1986 Nov;59(3):433–438. [PMC free article] [PubMed] [Google Scholar]
- Iturbe S., Narasimhan S., Letarte M. Structural analysis of the oligosaccharides of DR1 and DQw1 molecules. J Immunol. 1986 Jun 15;136(12):4596–4603. [PubMed] [Google Scholar]
- Iturbe S., Narasimhan S., Merrick J. M., Falk J. A., Letarte M. Comparison of the N-linked glycopeptides of DQw1 and DR1 molecules. J Immunol. 1986 Jun 15;136(12):4588–4595. [PubMed] [Google Scholar]
- Kaufman J. F., Strominger J. L. The extracellular region of light chains from human and murine MHC class II antigens consists of two domains. J Immunol. 1983 Feb;130(2):808–817. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Gogolin-Ewens K. J., Brandon M. R. Characterization of two sheep lymphocyte differentiation antigens, SBU-T1 and SBU-T6. Immunology. 1985 Aug;55(4):729–737. [PMC free article] [PubMed] [Google Scholar]
- Mengle-Gaw L., McDevitt H. O. Genetics and expression of mouse Ia antigens. Annu Rev Immunol. 1985;3:367–396. doi: 10.1146/annurev.iy.03.040185.002055. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F., Crumpton M. J. Biosynthesis and maturation of HLA-DR antigens in vivo. J Biol Chem. 1981 Sep 10;256(17):8987–8993. [PubMed] [Google Scholar]
- Puri N. K., Mackay C. R., Brandon M. R. Sheep lymphocyte antigens (OLA). II. Major histocompatibility complex class II molecules. Immunology. 1985 Dec;56(4):725–733. [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Kaufman J. F., Korman A. J., Strominger J. L. HLA-DR antigens: structure, separation of subpopulations, gene cloning and function. Immunol Rev. 1982;66:133–187. doi: 10.1111/j.1600-065x.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]