Abstract
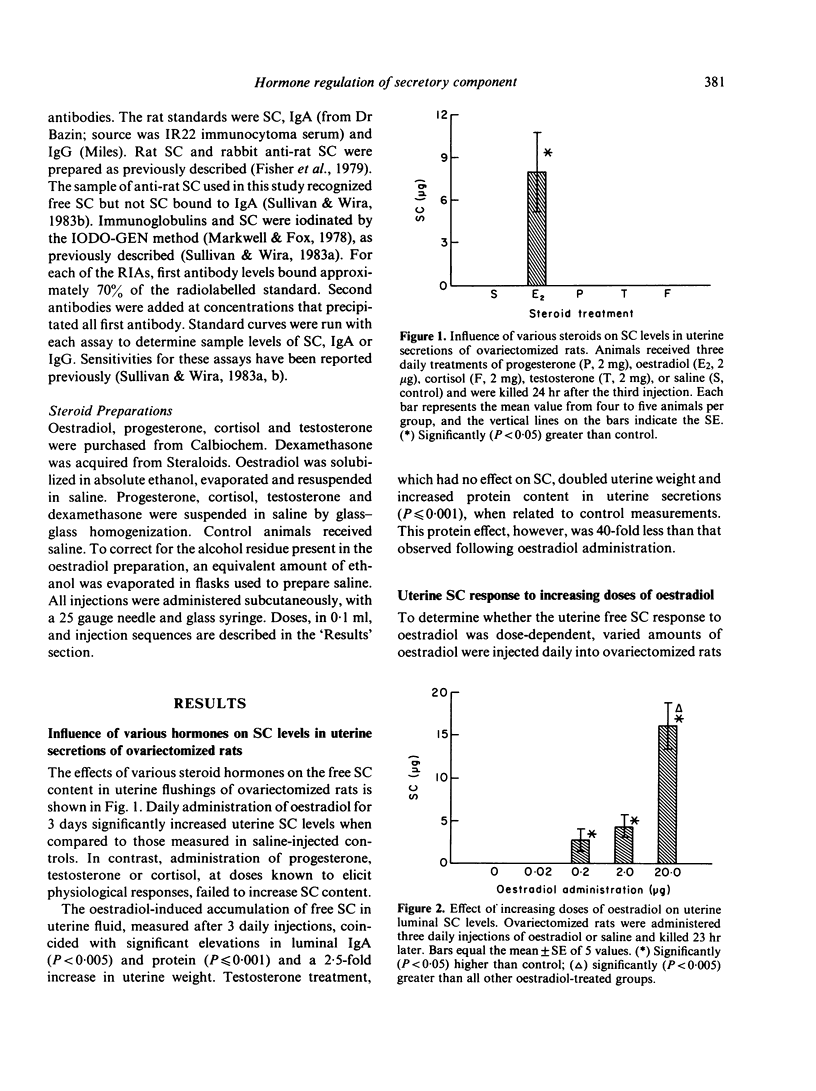
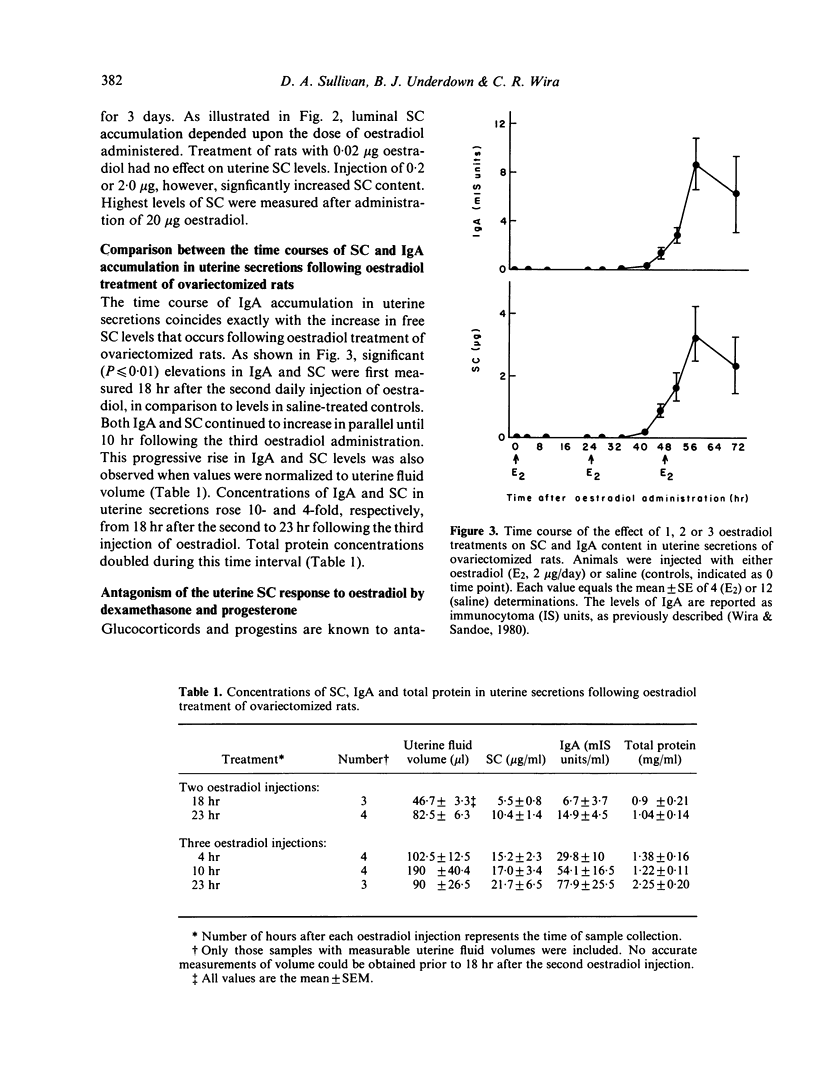
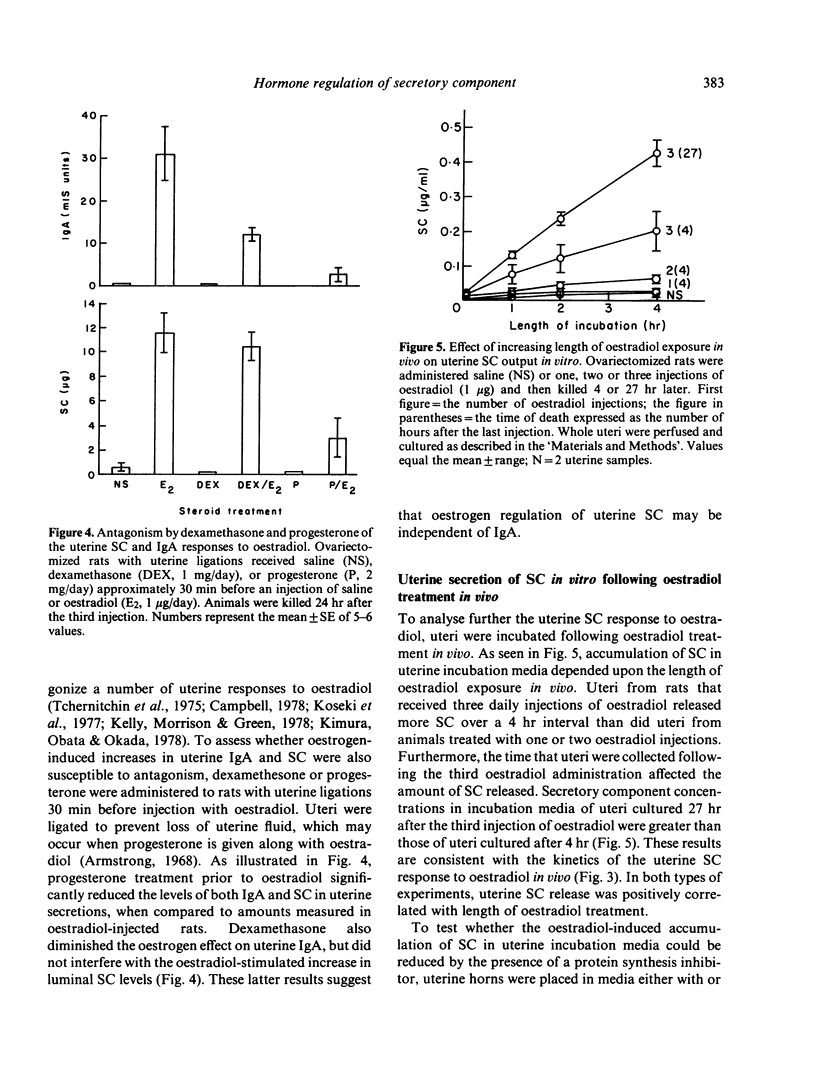
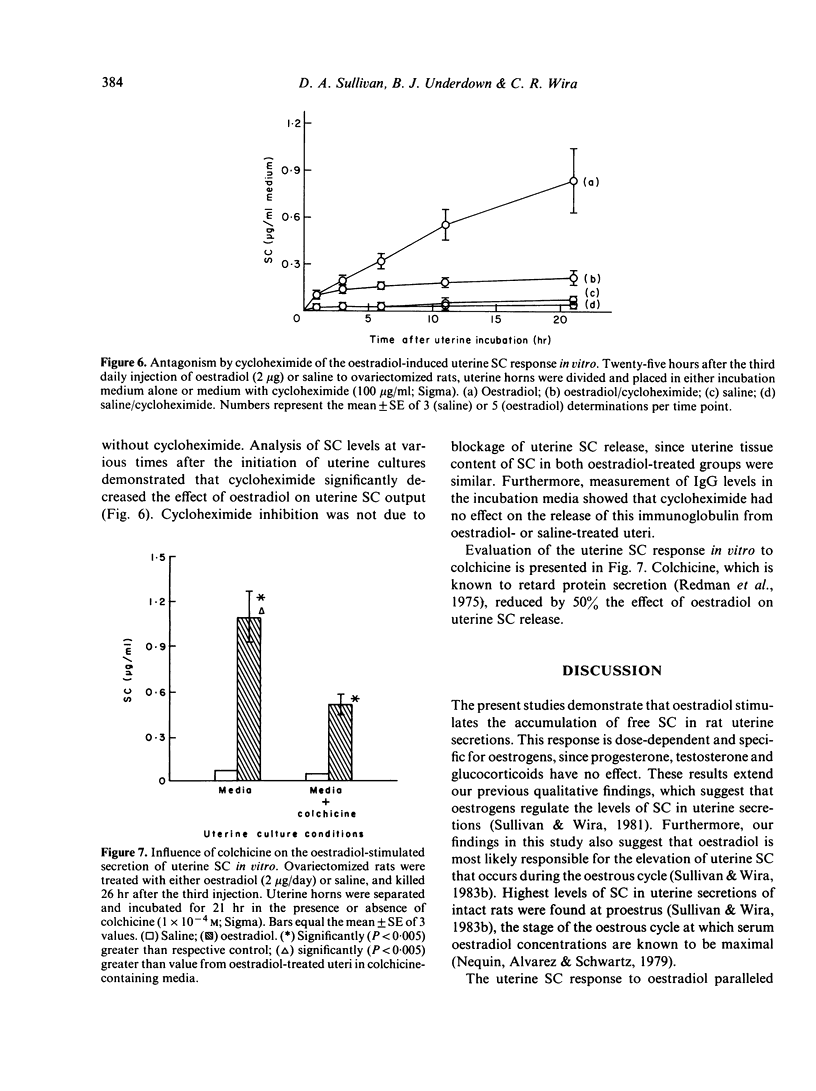
The present studies analysed the uterine free secretory component (SC) response to steroid hormones, and correlated effects on SC with those on IgA. Administration of oestradiol for 3 days to ovariectomized rats significantly increased the levels of SC in uterine secretions, when compared to those in saline-injected controls. This response was dose-dependent and specific for oestrogens, since progesterone, testosterone and glucocorticoids had no effect. The oestradiol-induced elevation in SC levels occurred in parallel with that of IgA. Time course studies of SC and IgA in uterine secretions indicated that both proteins accumulated in nearly identical patterns following oestradiol administration. The oestradiol-stimulated accumulation of SC, however, appears to be independent of IgA since dexamethasone treatment with oestradiol decreased IgA but not SC levels in uterine secretions. In contrast to dexamethasone, progesterone antagonized the oestradiol effect on both uterine IgA and SC. The uterine SC response to oestradiol was also observed in vitro. Incubation of uterine tissue following oestradiol exposure in vivo resulted in a significant accumulation of SC in the culture medium, when compared to levels from control uteri. Addition of either cycloheximide or colchicine to uterine incubation media significantly decreased the effect of oestradiol on SC accumulation. These results suggest that oestrogen regulation of uterine SC may involve stimulation of its synthesis. In addition, our findings indicate that oestradiol control of SC in uterine secretions may be responsible for the movement of IgA from uterine tissues to lumen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. T. Hormonal cohtrol of uterine lumen fluid retention in the rat. Am J Physiol. 1968 Apr;214(4):764–771. doi: 10.1152/ajplegacy.1968.214.4.764. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J Immunol. 1974 Apr;112(4):1553–1559. [PubMed] [Google Scholar]
- Brown W. R., Isobe K., Nakane P. K., Pacini B. Studies on translocation of immunoglobulins across intestinal epithelium. IV. Evidence for binding of IgA and IgM to secretory component in intestinal epithelium. Gastroenterology. 1977 Dec;73(6):1333–1339. [PubMed] [Google Scholar]
- Campbell P. S. The mechanism of the inhibition of uterotrophic responses by acute dexamethasone pretreatment. Endocrinology. 1978 Sep;103(3):716–723. doi: 10.1210/endo-103-3-716. [DOI] [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. R., Link D. W., Brown W. R., Nakane P. K. Ultrastructural evidence of transport of secretory IgA across bronchial epithelium. Am Rev Respir Dis. 1981 Jan;123(1):115–119. doi: 10.1164/arrd.1981.123.1.115. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kawashima K., Nakaura S., Nagao S., Tanaka S., Kuwamura T., Omori Y. Virilizing effect of methyltestosterone on female descendants in the rat. Endocrinol Jpn. 1978 Feb;25(1):1–6. doi: 10.1507/endocrj1954.25.1. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Morrison C., Green B. Effect of progesterone treatment on the uptake of estradiol-17 beta by uterine tissues of ovariectomised rats. Mol Cell Endocrinol. 1978 May;10(3):319–325. doi: 10.1016/0303-7207(78)90045-x. [DOI] [PubMed] [Google Scholar]
- Koseki Y., Zava D. T., Chamness G. C., McGuire W. L. Progesterone interaction with estrogen and antiestrogen in the rat uterus--receptor effects. Steroids. 1977 Aug;30(2):169–177. doi: 10.1016/0039-128x(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Lemaître-Coelho I., Altamirano G. A., Barranco-Acosta C., Meykens R., Vaerman J. P. In vivo experiments involving secretory component in the rat hepatic transfer of polymeric IgA from blood into bile. Immunology. 1981 Jun;43(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Jones R. S., Hinton R. H. Movement of endocytic shuttle vesicles from the sinusoidal to the bile canalicular face of hepatocytes does not depend on occupation of receptor sites. FEBS Lett. 1980 May 5;113(2):201–205. doi: 10.1016/0014-5793(80)80591-6. [DOI] [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Secretory component in immmunoglobulin deficiency: and immunoelectron microscopic study of intestinal epithelium. Scand J Immunol. 1980;12(4):359–363. doi: 10.1111/j.1365-3083.1980.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Translocation of dimeric IgA through neoplastic colon cells in vitro. J Immunol. 1979 Nov;123(5):2359–2368. [PubMed] [Google Scholar]
- Nequin L. G., Alvarez J., Schwartz N. B. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod. 1979 Apr;20(3):659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- Oldham G., Platts-Mills T. A., Chalmers D. M., Webster A. D. A quantitative method for measuring in vitro synthesis of IgA and IgG by human rectal mucosa: studies on normal controls and patients with hypogammaglobulinaemia. Immunology. 1979 Jul;37(3):661–668. [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. Colchicine inhibition of plasma protein release from rat hepatocytes. J Cell Biol. 1975 Jul;66(1):42–59. doi: 10.1083/jcb.66.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Estradiol regulation of secretory component in the female reproductive tract. J Steroid Biochem. 1981 Dec;15:439–444. doi: 10.1016/0022-4731(81)90311-3. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Hormonal regulation of immunoglobulins in the rat uterus: uterine response to a single estradiol treatment. Endocrinology. 1983 Jan;112(1):260–268. doi: 10.1210/endo-112-1-260. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Variations in free secretory component levels in mucosal secretions of the rat. J Immunol. 1983 Mar;130(3):1330–1335. [PubMed] [Google Scholar]
- Tchernitchin A., Rooryck J., Tchernitchin X., Vandenhende J., Galand P. Effects of cortisol on uterine eosinophilia and other oestrogenic responses. Mol Cell Endocrinol. 1975 May;2(5):331–337. doi: 10.1016/0303-7207(75)90020-9. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Hyde E., Sandoe C. P., Sullivan D., Spencer S. Cellular aspects of the rat uterine IgA response to estradiol and progesterone. J Steroid Biochem. 1980 Jan;12:451–459. doi: 10.1016/0022-4731(80)90306-4. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Sandoe C. P. Hormonal regulation of immunoglobulins: influence of estradiol on immunoglobulins A and G in the rat uterus. Endocrinology. 1980 Mar;106(3):1020–1026. doi: 10.1210/endo-106-3-1020. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Sandoe C. P. Sex steroid hormone regulation of IgA and IgG in rat uterine secretions. Nature. 1977 Aug 11;268(5620):534–536. doi: 10.1038/268534a0. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Sullivan D. A. Effect of estradiol and progesterone on the secretory immune system in the female genital tract. Adv Exp Med Biol. 1981;138:99–111. doi: 10.1007/978-1-4615-7192-6_6. [DOI] [PubMed] [Google Scholar]


