Abstract
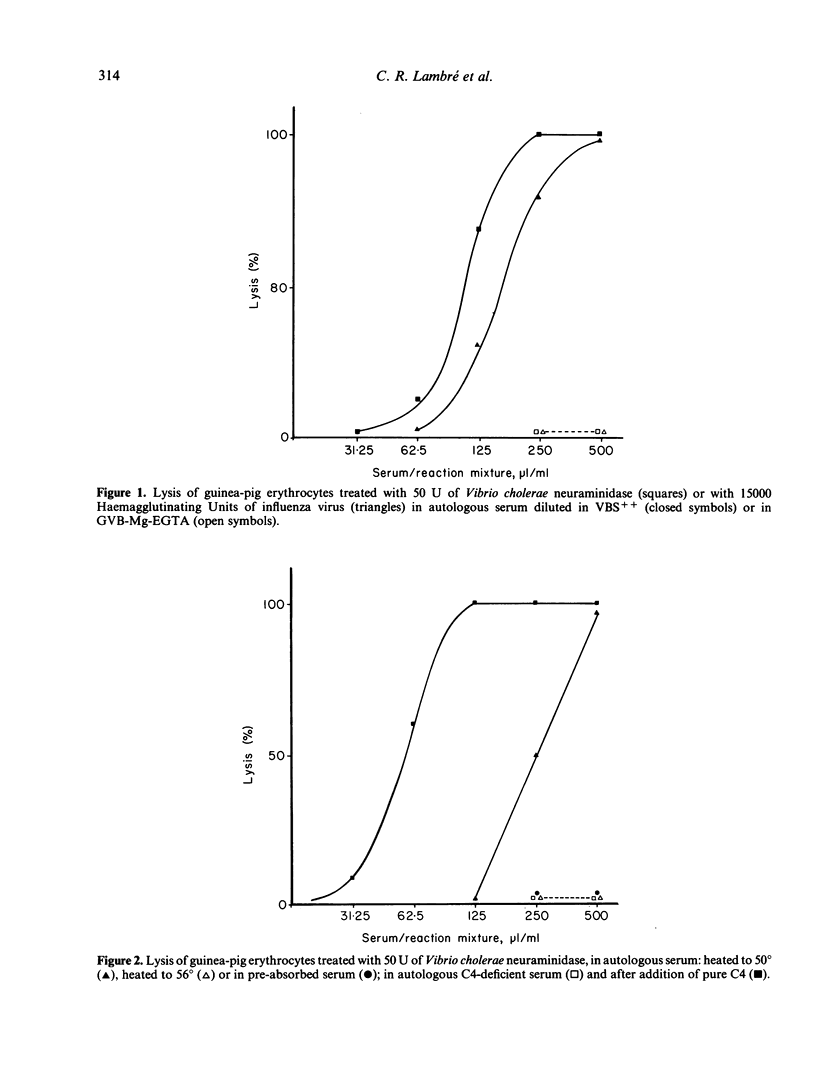
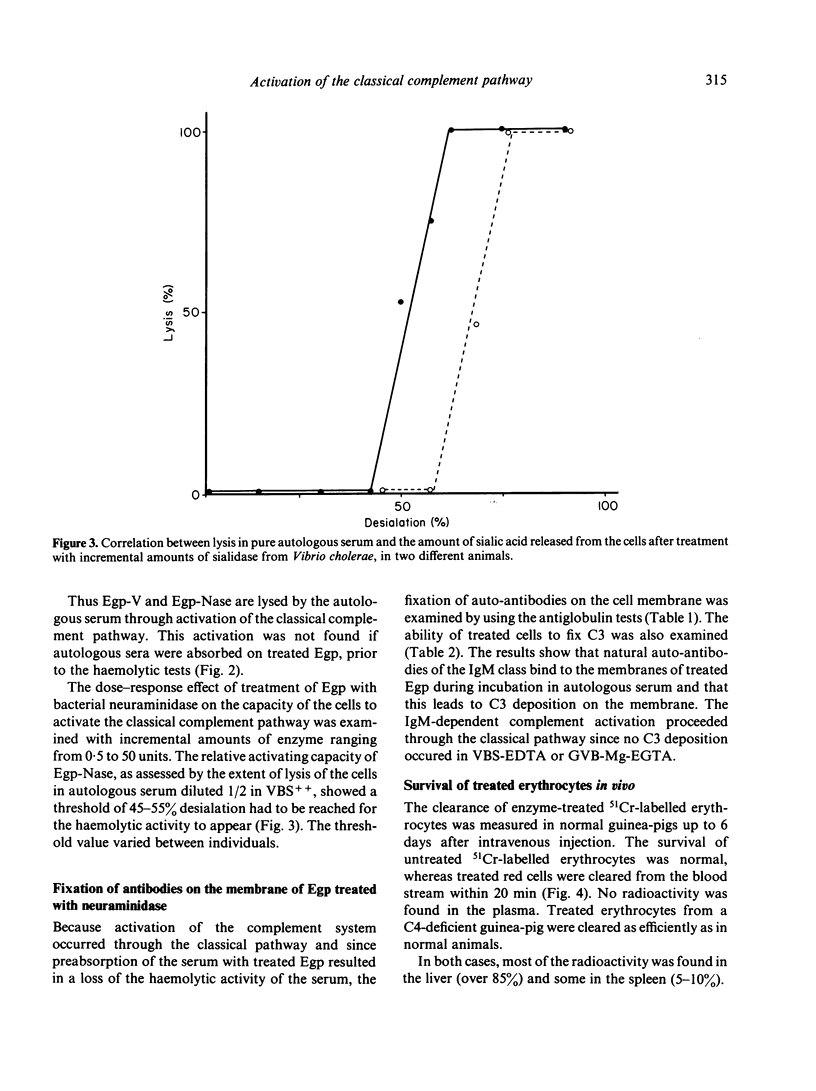
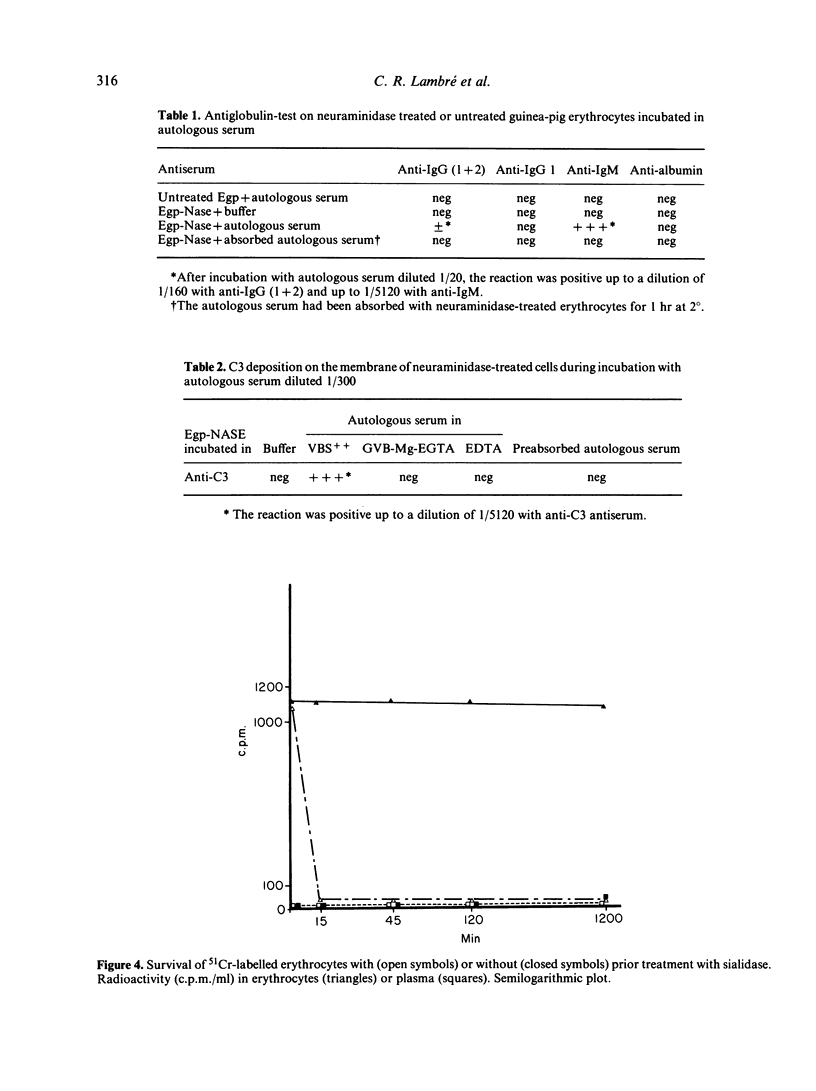
Guinea-pig erythrocytes that had been exposed to influenza A virus or Vibrio cholerae neuraminidase activated the classical complement pathway in autologous serum. Because all viral particles were eluted from the treated cells, activation was not dependent on anti-viral antibodies or on the particles themselves. After a threshold of 45-55% desialation, had been reached, the relative capacity of treated cells to activate complement increased very rapidly with desialation. Desialation unmasked sites on which natural auto-antibodies of the IgM class were fixed. Antibody fixation on the membrane led to C3b deposition on the cell membrane and activation of the classical complement sequence then cell lysis. The relevance of in vitro lysis of desialated cells to in vivo clearance of these cells is not certain because C4-deficient guinea-pigs were able to eliminate desialated cells from the blood stream as efficiently as did normal guinea-pigs. Nevertheless, membrane desialation occurring during myxovirus infection could lead to autoimmunity and tissue changes, as well as to recovery by eliminating virus-modified cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell W. C., Levy G. N., Williams R., Aminoff D. Effect of galactose oxidase, with and without prior sialidase treatment, on the viability of erythrocytes in circulation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4205–4209. doi: 10.1073/pnas.74.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Busse W. W., Cooper W., Warshauer D. M., Dick E. C., Wallow I. H., Albrecht R. Impairment of isoproterenol, H2 histamine, and prostaglandin E1 response of human granulocytes after incubation in vitro with live influenza vaccines. Am Rev Respir Dis. 1979 Apr;119(4):561–569. doi: 10.1164/arrd.1979.119.4.561. [DOI] [PubMed] [Google Scholar]
- Cabezas J. A., Calvo P., Eid P., Martin J., Perez E., Reglero A., Hannoun C. Neuraminidase from influenza virus A (H3N2): specificity towards several substrates and procedure of activity determination. Biochim Biophys Acta. 1980 Dec 4;616(2):228–238. doi: 10.1016/0005-2744(80)90141-2. [DOI] [PubMed] [Google Scholar]
- Desai U., Kreutzer D. L., Showell H., Arroyave C. V., Ward P. A. Acute inflammatory pulmonary reactions induced by chemotactic factors. Am J Pathol. 1979 Jul;96(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- GARDNER E., Jr, WRIGHT C. S., WILLIAMS B. Z. The survival of virus-treated erythrocytes in normal and splenectomized rabbits. J Lab Clin Med. 1961 Nov;58:743–750. [PubMed] [Google Scholar]
- Gattegno L., Bladier D., Cornillot P. The role of sialic acid in the determination of survival of rabbit erythrocytes in the circulation. Carbohydr Res. 1974 Jun;34(2):361–369. doi: 10.1016/s0008-6215(00)82911-0. [DOI] [PubMed] [Google Scholar]
- Hicks J. T., Ennis F. A., Kim E., Verbonitz M. The importance of an intact complement pathway in recovery from a primary viral infection: influenza in decomplemented and in C5-deficient mice. J Immunol. 1978 Oct;121(4):1437–1445. [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. Host modification of Sindbis virus sialic acid content influences alternative complement pathway activation and virus clearance. J Immunol. 1981 Nov;127(5):1740–1743. [PubMed] [Google Scholar]
- Hirsch R. L., Winkelstein J. A., Griffin D. E. The role of complement in viral infections. III. Activation of the classical and alternative complement pathways by Sindbis virus. J Immunol. 1980 May;124(5):2507–2510. [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974 Aug;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Lambre C., Kasturi K. N. A microplate immunoenzyme assay for anti-influenza antibodies. J Immunol Methods. 1979;26(1):61–67. doi: 10.1016/0022-1759(79)90041-3. [DOI] [PubMed] [Google Scholar]
- Lambré C. R., Kazatchkine M. D., Maillet F., Thibon M. Guinea pig erythrocytes, after their contact with influenza virus, acquire the ability to activate the human alternative complement pathway through virus-induced desialation of the cells. J Immunol. 1982 Feb;128(2):629–634. [PubMed] [Google Scholar]
- Lambré C., Thibon M. Ability to activate the alternative complement pathway acquired by human and guinea-pig erythrocytes after contact with influenza virus. Ann Immunol (Paris) 1980 Mar-Apr;131C(2):213–221. [PubMed] [Google Scholar]
- Larsen G. L., Mitchell B. C., Henson P. M. The pulmonary response of C5 sufficient and deficient mice to immune complexes. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):434–439. doi: 10.1164/arrd.1981.123.4.434. [DOI] [PubMed] [Google Scholar]
- Loos M., Brunner H. Complement components (C1, C2, C3, C4) in bronchial secretions after intranasal infection of guinea pigs with Mycoplasma pneumoniae: dissociation of unspecific and specific defense mechanisms. Infect Immun. 1979 Aug;25(2):583–585. doi: 10.1128/iai.25.2.583-585.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K., Henson P. M. Induction of lysosomal enzyme secretion by alveolar macrophages in response to the purified complement fragments C5a and C5a des-arg. J Immunol. 1979 Dec;123(6):2511–2517. [PubMed] [Google Scholar]
- McConnell I., Gorman N. T., Raniwalla J., Powell P. Activation of the alternative complement pathway by normal and transformed cells in homologous and heterologous sera. Eur J Immunol. 1981 Feb;11(2):126–132. doi: 10.1002/eji.1830110212. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Pickering R. J., Caliguiri L. A. Activation of the alternative complement pathway by enveloped viruses containing limited amounts of sialic acid. Virology. 1981 Oct 30;114(2):507–515. doi: 10.1016/0042-6822(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Kunkel S. L., Fantone J. C., Ward P. A. Comparative study of eosinophil and neutrophil chemotaxis and enzyme release. Am J Pathol. 1981 Nov;105(2):149–155. [PMC free article] [PubMed] [Google Scholar]
- Okada H., Okada N. Sendai virus infected cells are readily cytolysed by guinea-pig complement without antibody. Immunology. 1981 Jun;43(2):337–344. [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Acute viral infection: tissue injury mediated by anti-viral antibody through a complement effector system. J Immunol. 1971 Nov;107(5):1274–1280. [PubMed] [Google Scholar]
- Perrin L. H., Joseph B. S., Cooper N. R., Oldstone M. B. Mechanism of injury of virus-infected cells by antiviral antibody and complement: participation of IgG, F(ab')2, and the alternative complement pathway. J Exp Med. 1976 May 1;143(5):1027–1041. doi: 10.1084/jem.143.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J., Caldwell J. R., Castle J. R., Waldman R. H. Evidence for the presence of components of the alternative (properdin) pathway of complement activation in respiratory secretions. J Immunol. 1976 Sep;117(3):900–903. [PubMed] [Google Scholar]
- Rossen R. D., Butler W. T., Cate T. R., Szwed C. F., Couch R. B. Protein composition of nasal secretion during respiratory virus infection. Proc Soc Exp Biol Med. 1965 Aug-Sep;119(4):1169–1176. doi: 10.3181/00379727-119-30406. [DOI] [PubMed] [Google Scholar]
- STEWART W. B., PETENYI C. W., ROSE H. M. The survival time of canine erythrocytes modified by influenza virus. Blood. 1955 Mar;10(3):228–234. [PubMed] [Google Scholar]
- Shaw J. O., Henson P. M., Henson J., Webster R. O. Lung inflammation induced by complement-derived chemotactic fragments in the alveolus. Lab Invest. 1980 May;42(5):547–558. [PubMed] [Google Scholar]
- Smith T. F., McIntosh K., Fishaut M., Henson P. M. Activation of complement by cells infected with respiratory syncytial virus. Infect Immun. 1981 Jul;33(1):43–48. doi: 10.1128/iai.33.1.43-48.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Crook N. E., Streader L. G., Davidson B. E. The polypeptides of influenza virus. 8. Large-scale purification of the hemagglutinin. Virology. 1973 Dec;56(2):640–645. doi: 10.1016/0042-6822(73)90066-4. [DOI] [PubMed] [Google Scholar]
- Stimler N. P., Hugli T. E., Bloor C. M. Pulmonary injury induced by C3a and C5a anaphylatoxins. Am J Pathol. 1980 Aug;100(2):327–348. [PMC free article] [PubMed] [Google Scholar]
- Verbonitz M. W., Ennis F. A., Hicks J. T., Albrecht P. Hemagglutinin-specific complement-dependent cytolytic antibody response to influenza infection. J Exp Med. 1978 Jan 1;147(1):265–270. doi: 10.1084/jem.147.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


