Abstract
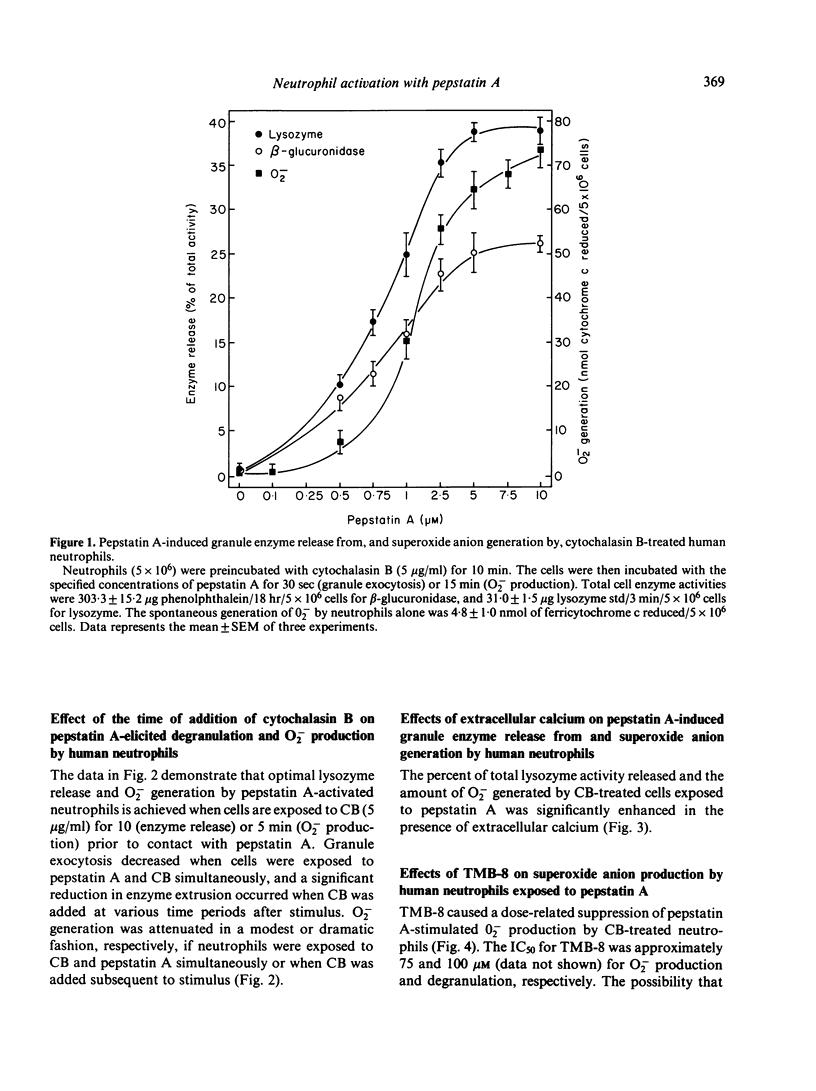
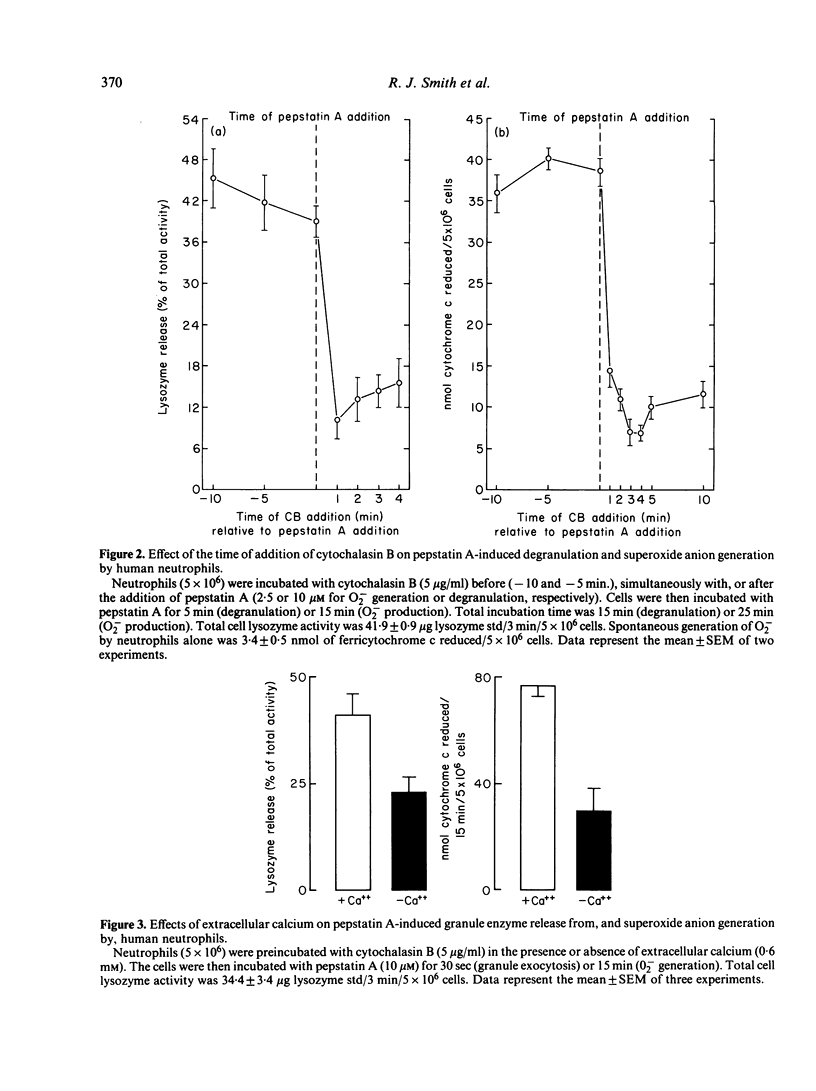
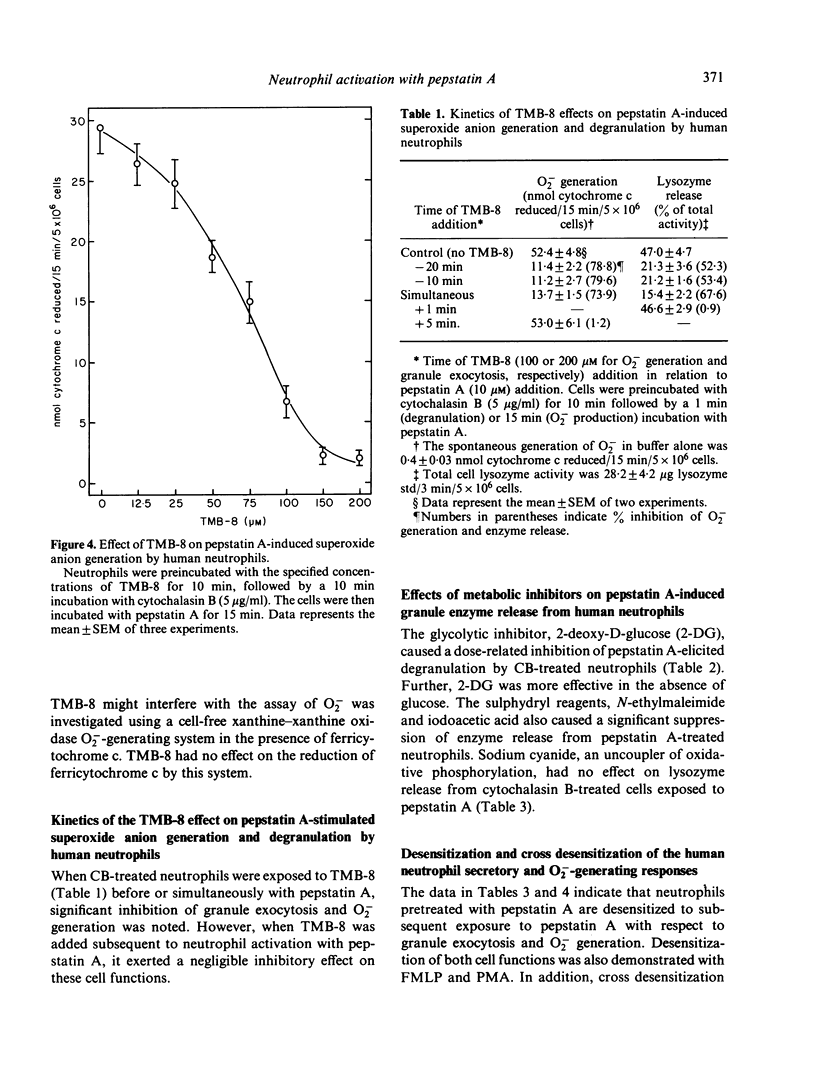
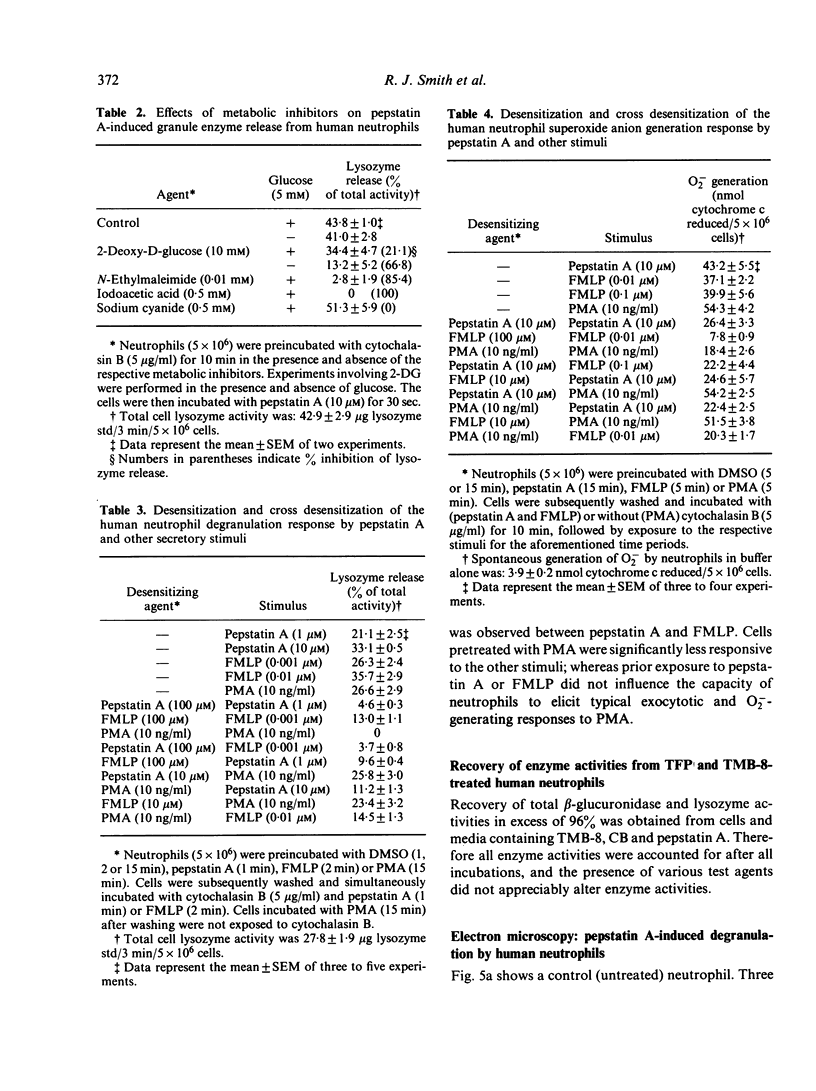
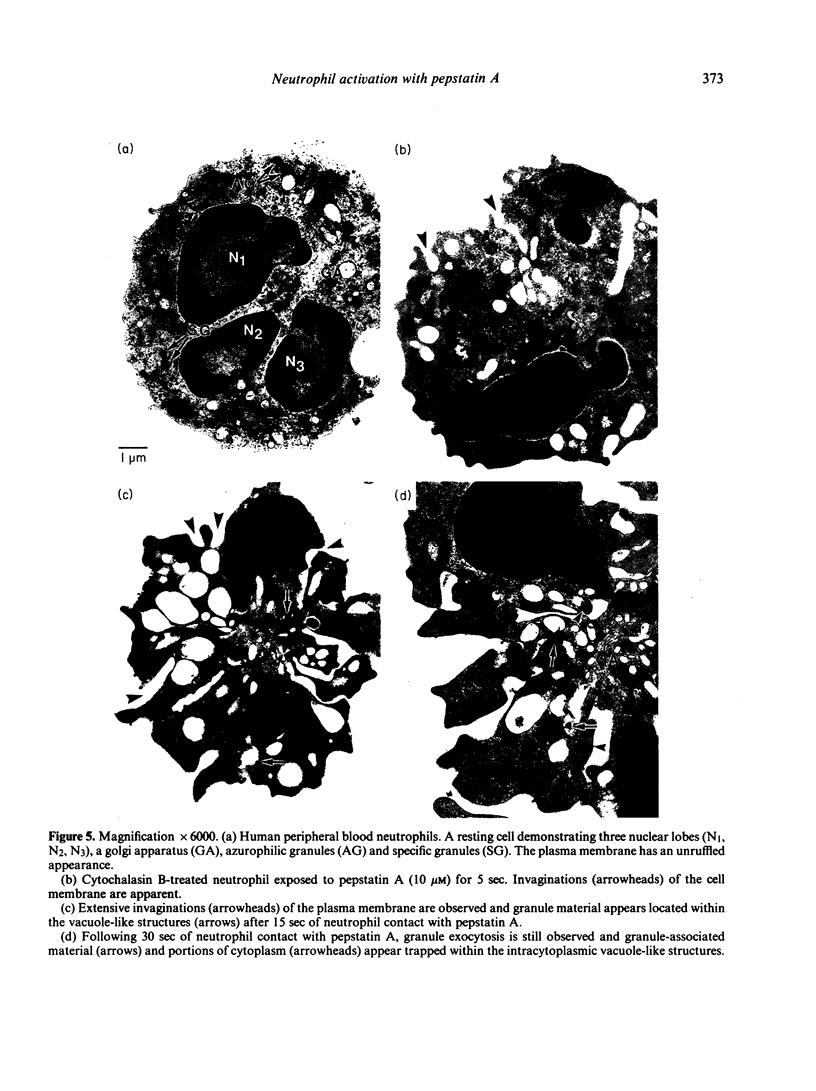
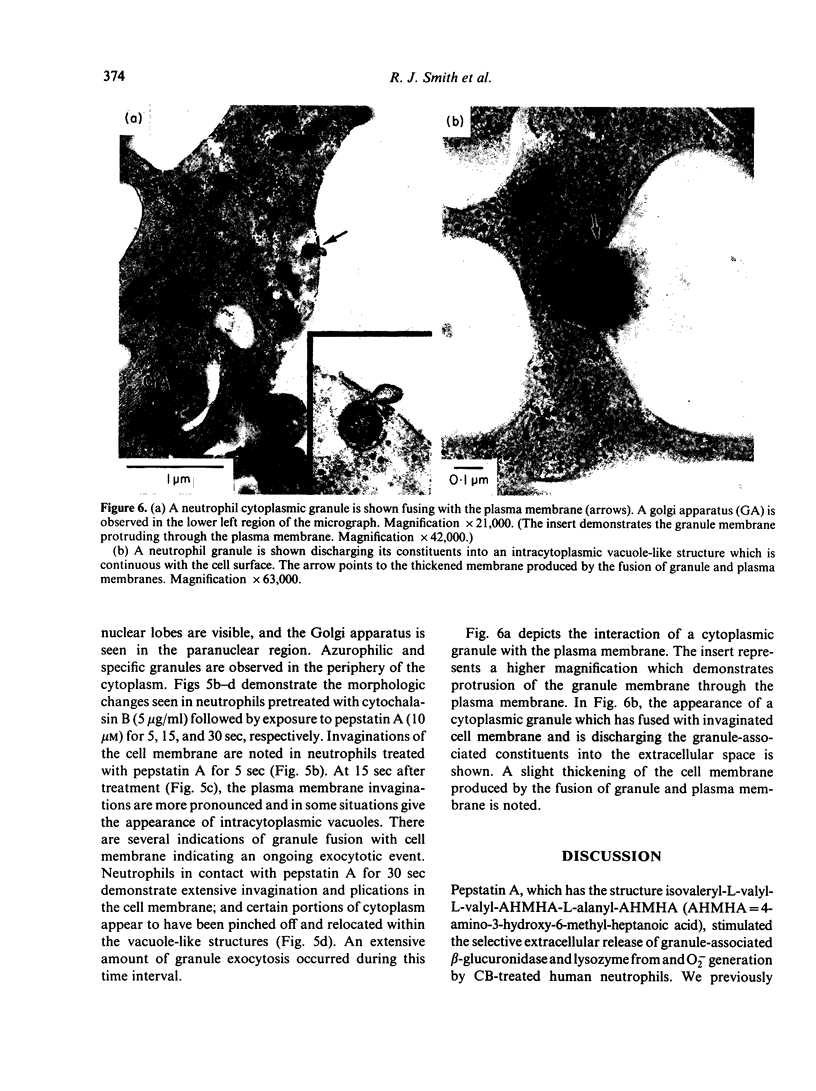
Pepstatin A, a chemotactic pentapeptide, elicited a concentration-dependent extracellular release of granule-associated beta-glucuronidase and lysozyme from, and generation of superoxide anion (O2-) by, cytochalasin B (CB)-treated human neutrophils. Prior exposure of neutrophils to pepstatin A before the addition of CB, suppressed, in a time-dependent fashion, the subsequent production of O2- and exocytotic response. The rate and amount of enzymes released and O2- generated by pepstatin A-activated neutrophils were significantly enhanced in the presence of extracellular calcium. Pepstatin A-elicited degranulation and O2- production were suppressed by the intracellular calcium antagonist, 8-(N,N-diethylamino)-octyl-(3, 4, 5-trimethoxy) benzoate hydrochloride (TMB-8). Granule exocytosis and O2- generation by pepstatin A-treated neutrophils were suppressed by the sulphydryl reagents, N-ethylmaleimide (NEM) and iodoacetic acid (IA), and by the glycolytic inhibitor, 2-deoxy-D-glucose (2-DG). Sodium cyanide was inactive. Preincubation of neutrophils with pepstatin A "desensitized' the cells to a subsequent exposure to pepstatin A or the chemotactic tripeptide, N-formyl-methionyl-leucyl-phenylalanine (FMLP). Pepstatin A-induced desensitization of granule enzyme release and O2- generation appears to be stimulus-specific in that phorbol myristate acetate (PMA) was capable of eliciting normal responses from pepstatin A-pretreated cells. The morphological changes observed in pepstatin A-treated neutrophils are reminiscent of those seen in cells exposed to FMLP.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Pepstatin A--a human leukocyte chemoattractant. Clin Immunol Immunopathol. 1979 Oct;14(2):244–250. doi: 10.1016/0090-1229(79)90146-6. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Becker E. L., Showell H. J. The ability of chemotactic factors to induce lysosomal enzyme release. II. The mechanism of release. J Immunol. 1974 Jun;112(6):2055–2062. [PubMed] [Google Scholar]
- Henson P. M. Mechanisms of exocytosis in phagocytic inflammatory cells. Parke-Davis Award Lecture. Am J Pathol. 1980 Dec;101(3):494–511. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Schwartzman N. A., Zanolari B. Intracellular control of human neutrophil secretion. II. Stimulus specificity of desensitization induced by six different soluble and particulate stimuli. J Immunol. 1981 Aug;127(2):754–759. [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Hoffstein S., Weissmann G. Microfilaments and microtubules in calcium ionophore-induced secretion of lysosomal enzymes from human polymorphonuclear leukocytes. J Cell Biol. 1978 Sep;78(3):769–781. doi: 10.1083/jcb.78.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo C., Lefkowitz R. J., Snyderman R. The oligopeptide chemotactic factor receptor on human polymorphonuclear leukocyte membranes exists in two affinity states. Biochem Biophys Res Commun. 1982 May 31;106(2):442–449. doi: 10.1016/0006-291x(82)91130-5. [DOI] [PubMed] [Google Scholar]
- Mackin W. M., Huang C. K., Becker E. L. The formylpeptide chemotactic receptor on rabbit peritoneal neutrophils. I. Evidence for two binding sites with different affinities. J Immunol. 1982 Oct;129(4):1608–1611. [PubMed] [Google Scholar]
- Malagodi M. H., Chiou C. Y. Pharmacological evaluation of a new Ca2+ antagonist, 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in smooth muscles. Eur J Pharmacol. 1974 Jun;27(1):25–33. doi: 10.1016/0014-2999(74)90198-8. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Ackerman S. K., Fiegel V. D., Bauman M. P., Douglas S. D. Cytotaxin receptors of neutrophils: evidence that F-methionyl peptides and pepstatin share a common receptor. Infect Immun. 1979 Dec;26(3):996–999. doi: 10.1128/iai.26.3.996-999.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Williams D., Becker E. L., Naccache P. H., Sha'afi R. Desensitization and deactivation of the secretory responsiveness of rabbit neutrophils induced by the chemotactic peptide, formyl-methionyl-leucyl-phenylalanine. J Reticuloendothel Soc. 1979 Feb;25(2):139–150. [PubMed] [Google Scholar]
- Smith R. J., Bowman B. J., Iden S. S. Pepstatin A-induced degranulation and superoxide anion generation by human neutrophils. Clin Immunol Immunopathol. 1982 Jan;22(1):83–93. doi: 10.1016/0090-1229(82)90025-3. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Modulation of human neutrophil superoxide anion generation by the calcium antagonist 8-(N,N-diethylamino)-octyl-(3,4,5-trimethoxy) benzoate hydrochloride. J Reticuloendothel Soc. 1981 Mar;29(3):215–225. [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Phorbol myristate acetate-induced release of granule enzymes from human neutrophils: inhibition by the calcium antagonist, 8-(N,N-diethylamino)-octyl 3,4,5-trimethoxybenzoate hydrochloride. Biochem Biophys Res Commun. 1979 Nov 14;91(1):263–271. doi: 10.1016/0006-291x(79)90612-0. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Properties of calcium ionophore-induced generation of superoxide anion by human neutrophils. Inflammation. 1981 Sep;5(3):177–192. doi: 10.1007/BF00914442. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Properties of concanavalin A-elicited granule exocytosis from human polymorphonuclear neutrophils. Inflammation. 1980 Dec;4(4):343–358. doi: 10.1007/BF00916046. [DOI] [PubMed] [Google Scholar]
- Smith R. J. Modulation of phagocytosis by and lysosomal enzyme secretion from guinea-pig neutrophils: effect of nonsteroid anti-inflammatory agents and prostaglindins. J Pharmacol Exp Ther. 1977 Mar;200(3):647–657. [PubMed] [Google Scholar]
- Smith R. J., Sabin C., Gilchrest H., Williams S. Effect of anti-inflammatory drugs on lysosomes and lysosomal enzymes from rat liver. Biochem Pharmacol. 1976 Oct 1;25(19):2171–2177. doi: 10.1016/0006-2952(76)90129-5. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Morishima H., Matsuzaki M., Hamada M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot (Tokyo) 1970 May;23(5):259–262. doi: 10.7164/antibiotics.23.259. [DOI] [PubMed] [Google Scholar]