Abstract
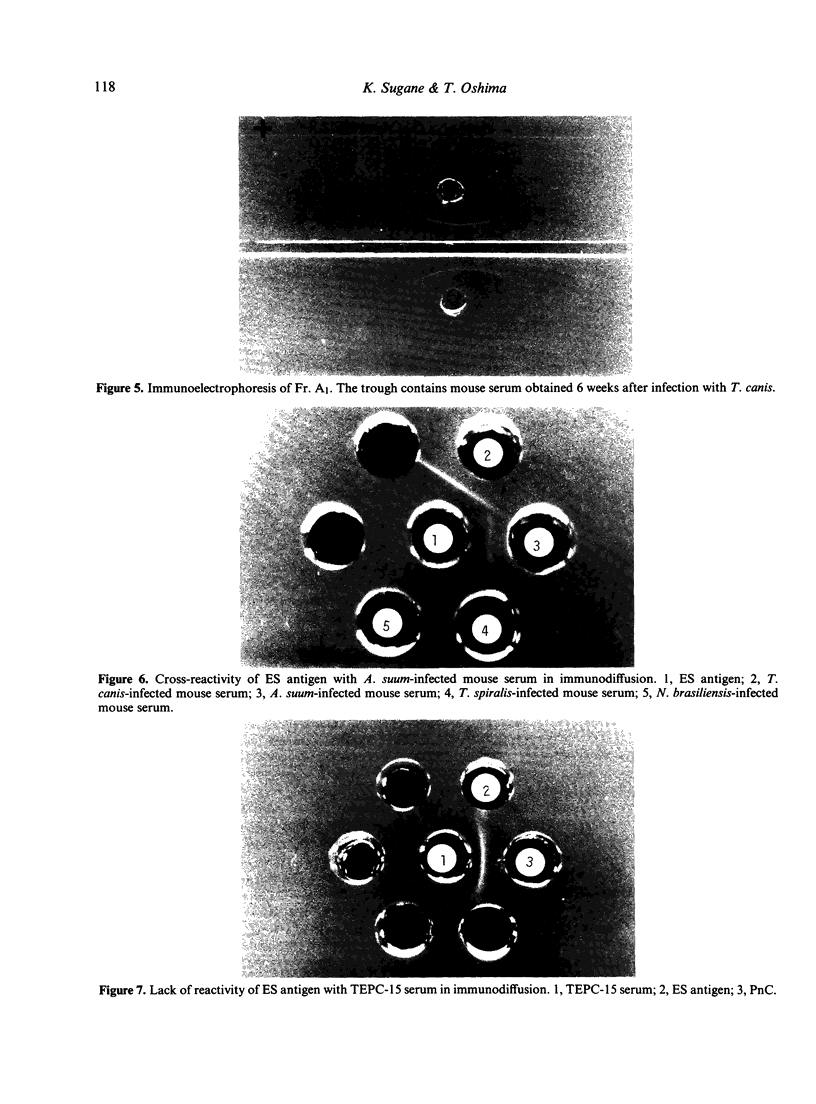
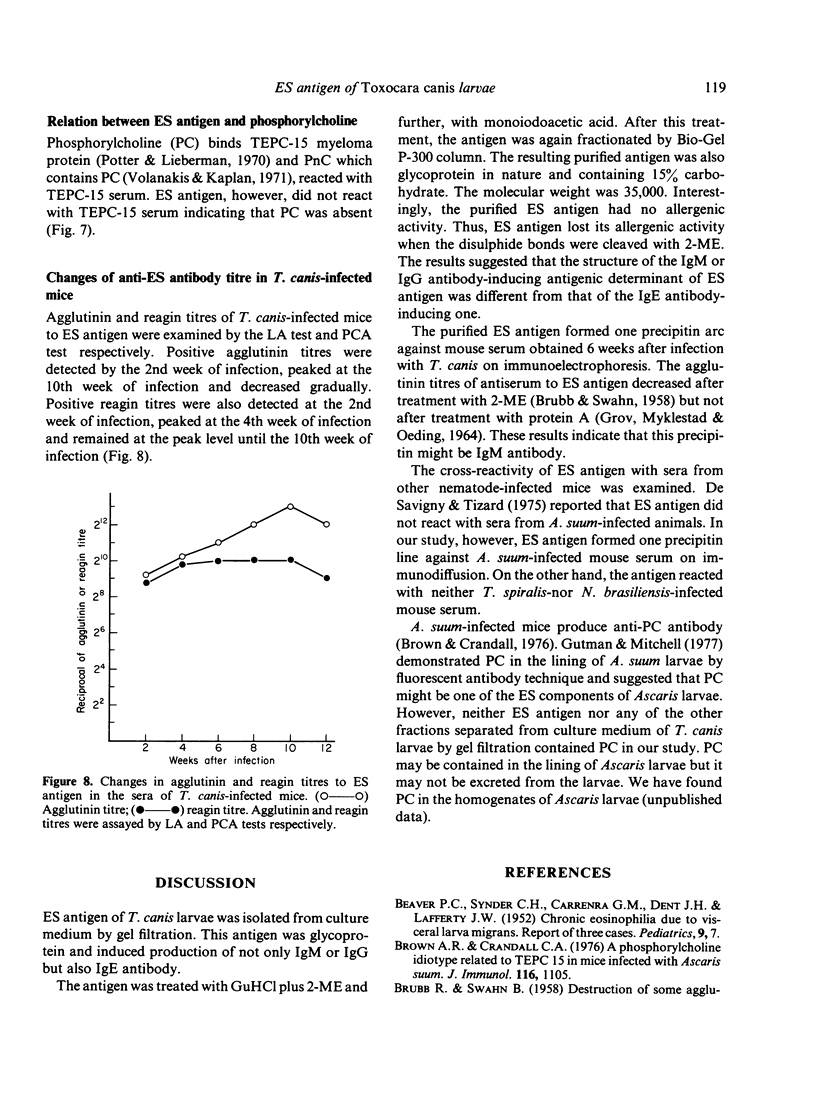
Excretory/secretory (ES) antigen of Toxocara canis larvae was isolated from the culture medium of second-stage larvae by gel filtration. The antigen induced production of not only IgM or IgG but also IgE antibody. The antigen, when treated with guanidine hydrochloride and 2-mercaptoethanol, lost its allergenic activity but retained an ability to induce IgM or IgG antibody. Purified antigen was of glycoprotein nature and had a molecular weight of 35,000. The whole antigen showed a cross-reaction with the serum from Ascaris suum-infected mice. The antigen did not contain phosphorylcholine as a structural component.
Full text
PDF


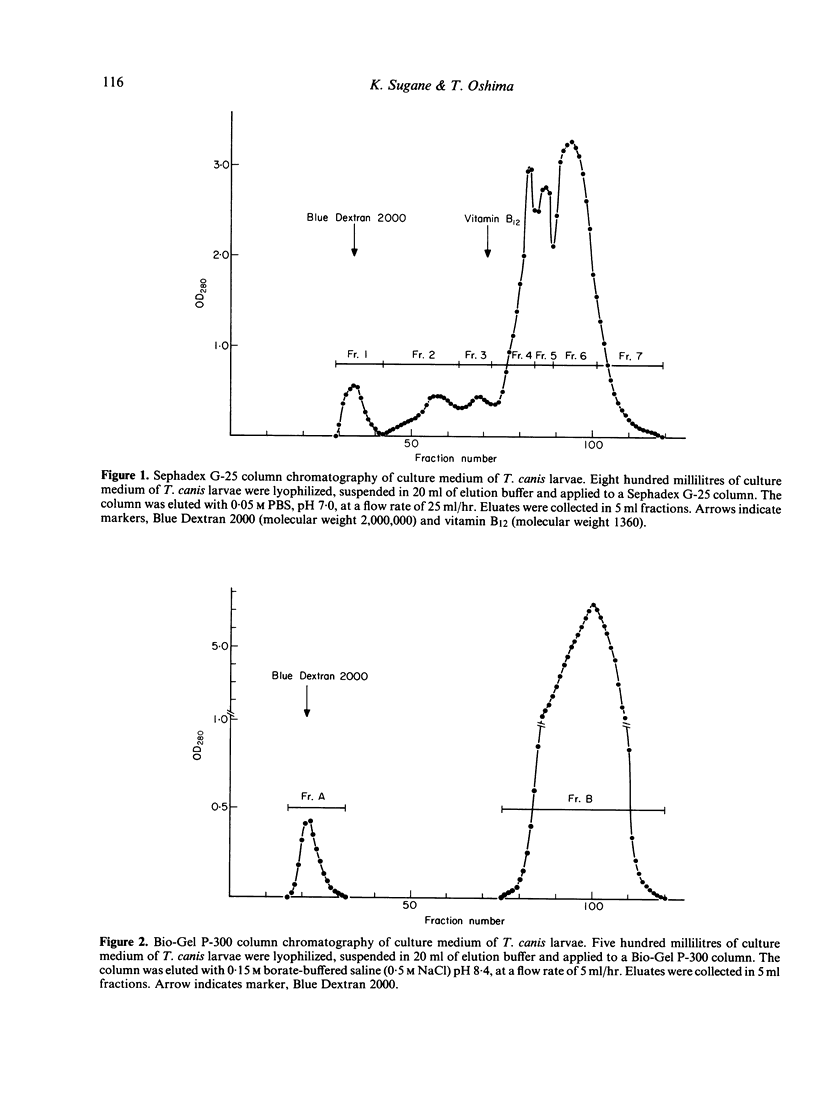
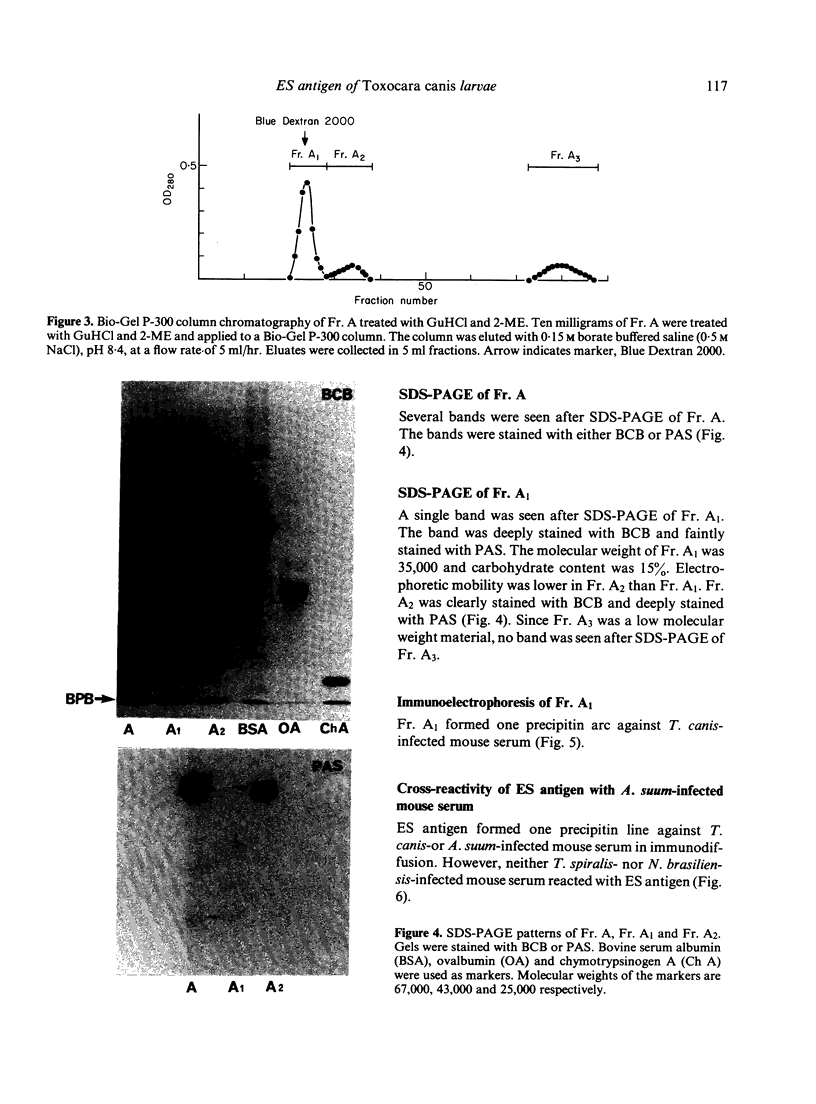

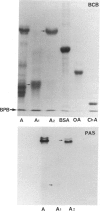
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. R., Crandall C. A. A phosphorylcholine idiotype related to TEPC 15 in mice infected with Ascaris suum. J Immunol. 1976 Apr;116(4):1105–1109. [PubMed] [Google Scholar]
- Fujita K., Tsukidate S. Preparation of a highly purified allergen from Dirofilaria immitis. Reaginic antibody formation in mice. Immunology. 1981 Mar;42(3):363–370. [PMC free article] [PubMed] [Google Scholar]
- GROV A., MYKLESTAD B., OEDING P. IMMUNOCHEMICAL STUDIES ON ANTIGEN PREPARATIONS FROM STAPHYLOCOCCUS AUREUS. 1. ISOLATION AND CHEMICAL CHARACTERIZATION OF ANTIGEN A. Acta Pathol Microbiol Scand. 1964;61:588–596. doi: 10.1111/apm.1964.61.4.588. [DOI] [PubMed] [Google Scholar]
- Grove D. I., Mahmoud A. A., Warren K. S. Eosinophils and resistance to Trichinella spiralis. J Exp Med. 1977 Mar 1;145(3):755–759. doi: 10.1084/jem.145.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman G. A., Mitchell G. F. Ascaris suum: location of phosphorylcholine in lung larvae. Exp Parasitol. 1977 Oct;43(1):161–168. doi: 10.1016/0014-4894(77)90019-4. [DOI] [PubMed] [Google Scholar]
- Krupp I. M. Hemagglutination test for the detection of antibodies specific for Ascaris and Toxocara antigens in patients with suspected visceral larva migrans. Am J Trop Med Hyg. 1974 May;23(3):378–384. doi: 10.4269/ajtmh.1974.23.378. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- OLSON L. J. Organ disturbution of Toxocara canis larvae in normal mice and in mice previously infected with Toxocara, Ascaris or Trichinella. Tex Rep Biol Med. 1962;20:651–657. [PubMed] [Google Scholar]
- OSHIMA T. Standardization of techniques for infecting mice with Toxocara canis and observations on the normal migration routes of the larvae. J Parasitol. 1961 Aug;47:652–656. [PubMed] [Google Scholar]
- Potter M., Lieberman R. Common individual antigenic determinants in five of eight BALB-c IgA myeloma proteins that bind phosphoryl choline. J Exp Med. 1970 Oct 1;132(4):737–751. doi: 10.1084/jem.132.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER J. M., PLOTZ C. M., PADER E., ELSTER S. K. The latex-fixation test. III. Agglutination test for C-reactive protein and comparison with the capillary precipitin method. Am J Clin Pathol. 1957 Dec;28(6):611–617. doi: 10.1093/ajcp/28.6.611. [DOI] [PubMed] [Google Scholar]
- Savigny D. H. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J Parasitol. 1975 Aug;61(4):781–782. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sugane K., Oshima T. Recovery of large numbers of eosinophils from mice infected with Toxocara canis. Am J Trop Med Hyg. 1980 Sep;29(5):799–802. doi: 10.4269/ajtmh.1980.29.799. [DOI] [PubMed] [Google Scholar]
- Volanakis J. E., Kaplan M. H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971 Feb;136(2):612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]