Abstract
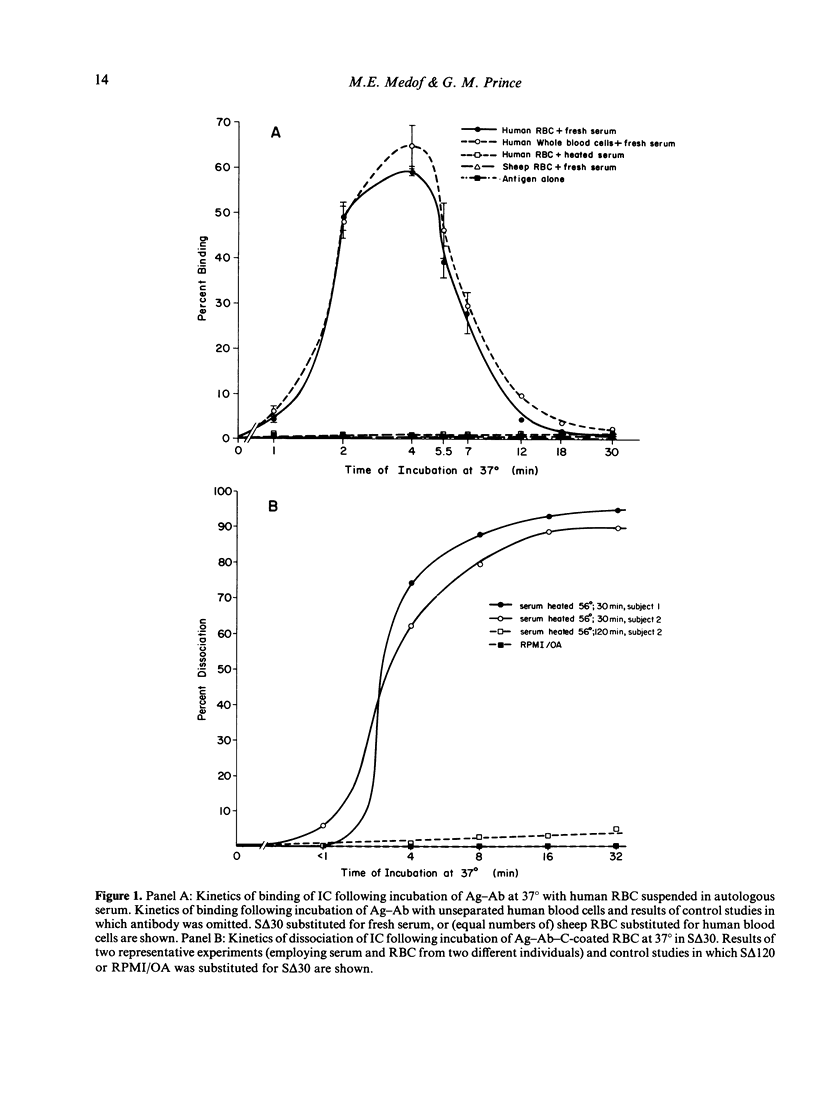
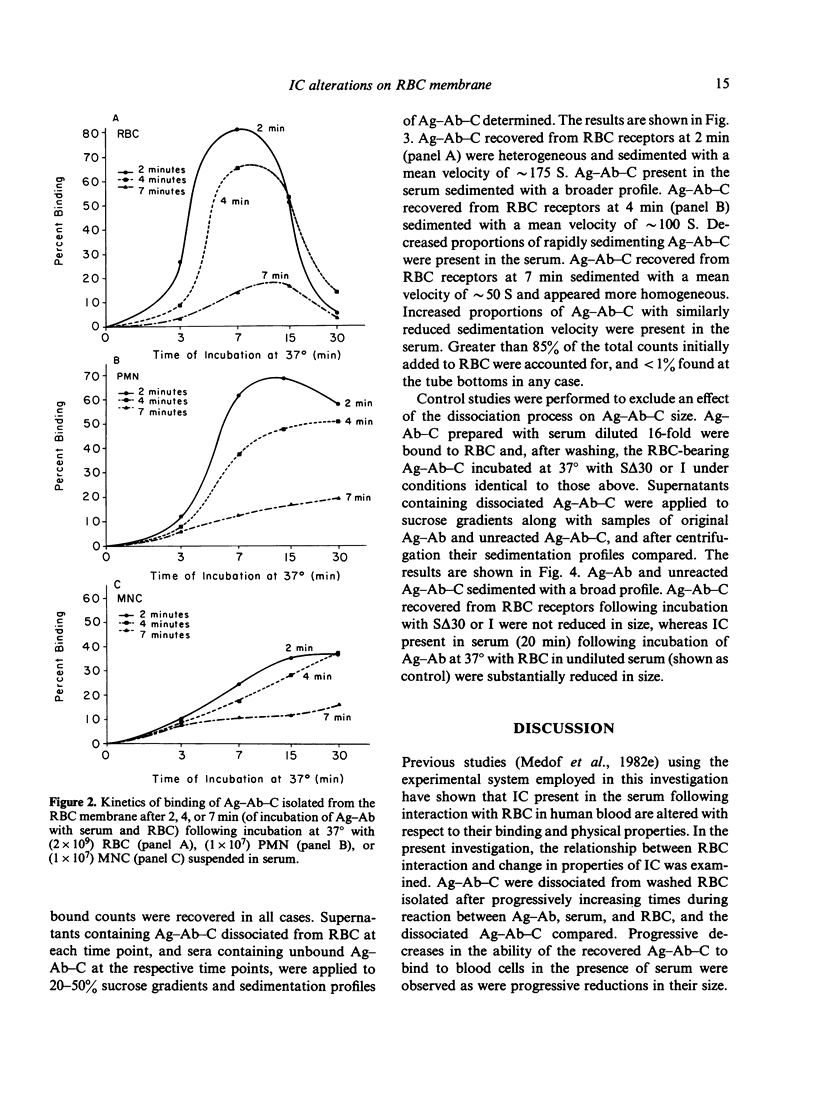
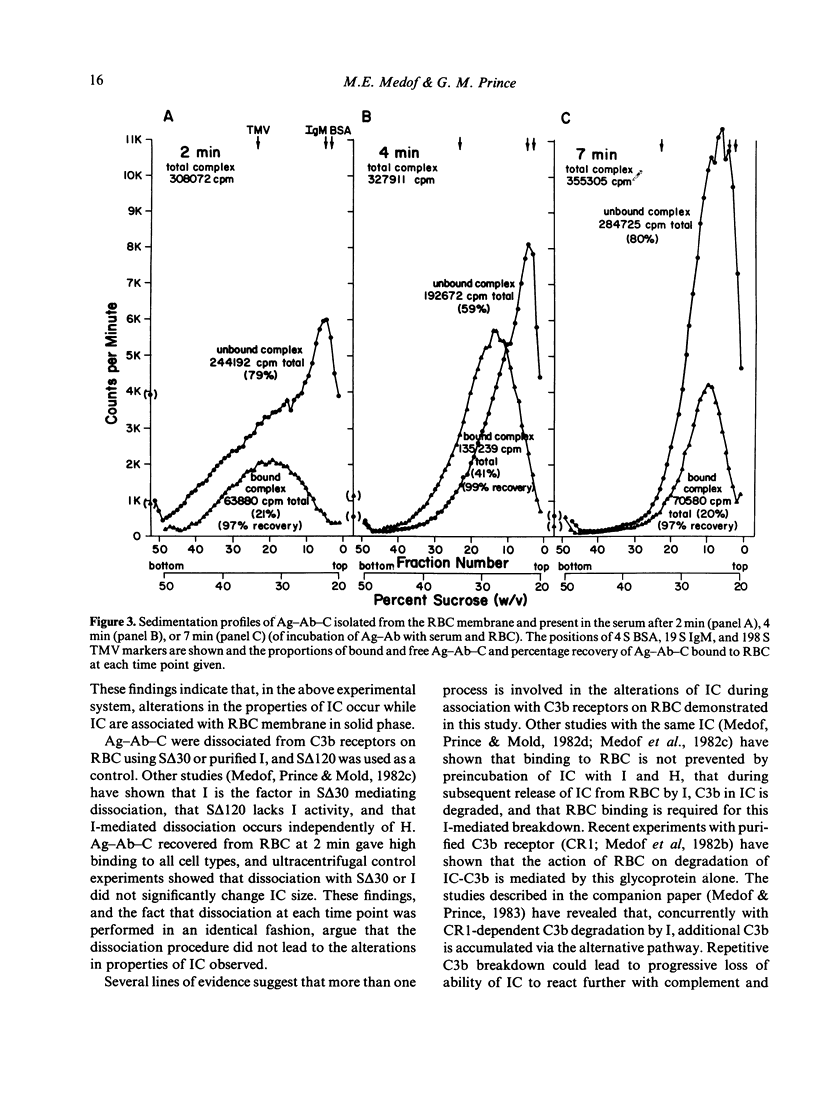
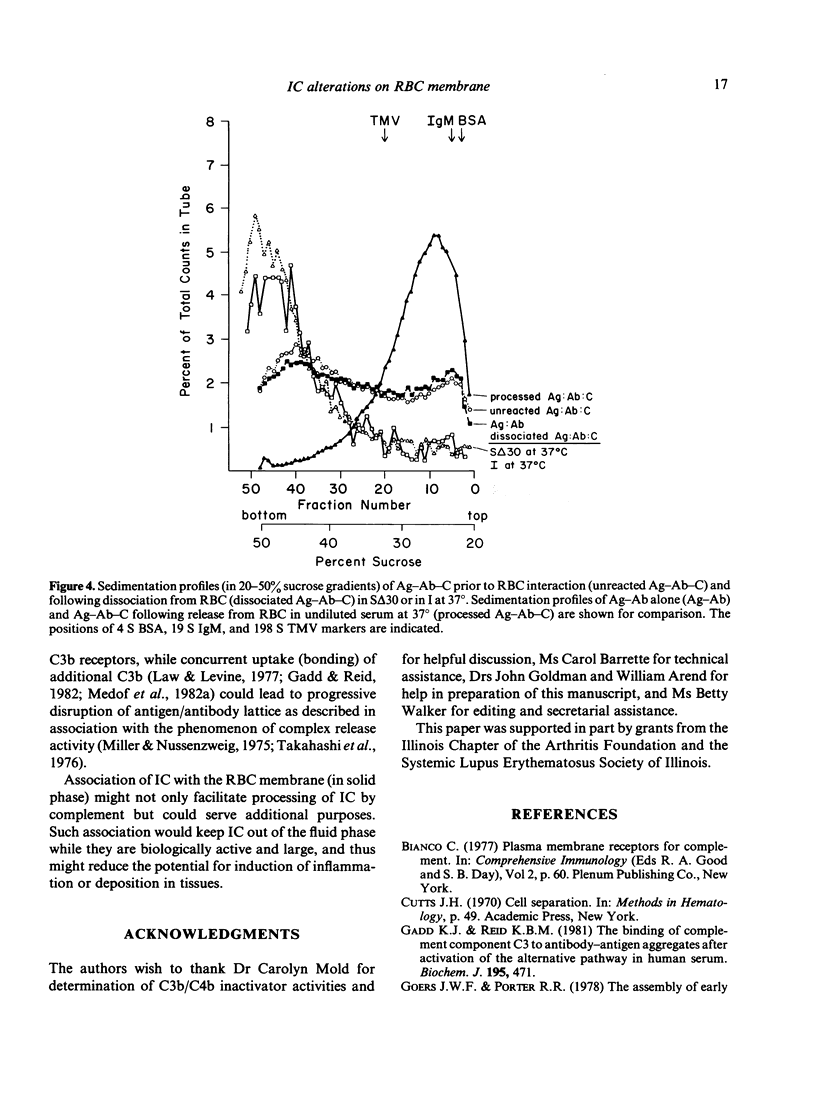
Experimental antigen-antibody complexes (Ag-Ab) were incubated at 37 degrees with human red blood cells (RBC) suspended in autologous normal serum and the reaction stopped after progressively increasing times. Bound antigen-antibody-complement complexes (Ag-Ab-C) were eluted from C3b receptors and the eluted Ag-Ab-C re-incubated with different blood cell types suspended in serum, or centrifuged (along with unbound Ag-Ab-C found in the serum) through 20-50% sucrose gradients. Ag-Ab-C recovered from C3b receptors shortly after initial binding to RBC bound efficiently to other RBC, polymorphonuclear and mononuclear cells, and sedimented rapidly. Ag-Ab-C simultaneously present in the serum sedimented with a similar velocity. Ag-Ab-C recovered at a subsequent time during RBC interaction bound less well to each blood cell type, and sedimented less rapidly. Decreased amounts of rapidly sedimenting Ag-Ab-C were present in the serum. Ag-Ab-C recovered from C3b receptors at a still later time in the course of RBC interaction bound poorly to each cell type, and sedimented slowly. Increased amounts of slowly sedimenting Ag-Ab-C were found in the serum. These findings indicate that alterations in properties of immune complexes can occur while they are associated with C3b receptors on RBC membrane in solid phase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gadd K. J., Reid K. B. The binding of complement component C3 to antibody-antigen aggregates after activation of the alternative pathway in human serum. Biochem J. 1981 May 1;195(2):471–480. doi: 10.1042/bj1950471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goers J. W., Porter R. R. The assembly of early components of complement on antibody-antigen aggregates and on antibody-coated erythrocytes. Biochem J. 1978 Nov 1;175(2):675–684. doi: 10.1042/bj1750675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Oger J. J. Competition for immune complexes by red cells in human blood. J Clin Lab Immunol. 1982 Jan;7(1):7–13. [PubMed] [Google Scholar]
- Medof M. E., Prince G. M., Mold C. Release of soluble immune complexes from immune adherence receptors on human erythrocytes is mediated by C3b inactivator independently of Beta 1H and is accompanied by generation of C3c. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5047–5051. doi: 10.1073/pnas.79.16.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. W., Nussenzweig V. A new complement function: solubilization of antigen-antibody aggregates. Proc Natl Acad Sci U S A. 1975 Feb;72(2):418–422. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Czop J., Ferreira A., Nussenzweig V. Mechanism of solubilization of immune aggregates by complement. Implications for immunopathology. Transplant Rev. 1976;32:121–139. doi: 10.1111/j.1600-065x.1976.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


