Abstract
The activities of many prokaryotic σ54-dependent transcriptional activators are controlled by the N-terminal A-domain of the protein, which is linked to the central transcriptional activation domain via a short B-linker. It used to be thought that these B-linkers simply serve as flexible tethers. Here we show that the B-linker of the aromatic-responsive regulator DmpR and many other regulators of the family contain signature heptad repeats with regularly spaced hydrophobic amino acids. Mutant analysis of this region of DmpR demonstrates that B-linker function is dependent on the heptad repeats and is critical for activation of the protein by aromatic effectors. The phenotypes of DmpR mutants refute the existing model that the level of ATPase activity directly controls the level of transcription it promotes. The mutant analysis also shows that the B-linker is involved in repression of ATPase activity and that allosteric changes upon effector binding are transduced to alleviate both B-linker repression of ATP hydrolysis and A-domain repression of transcriptional activation. The mechanistic implications of these findings for DmpR and other family members are discussed.
Keywords: coiled coil/DmpR/linker/σ54/transcriptional activation
Introduction
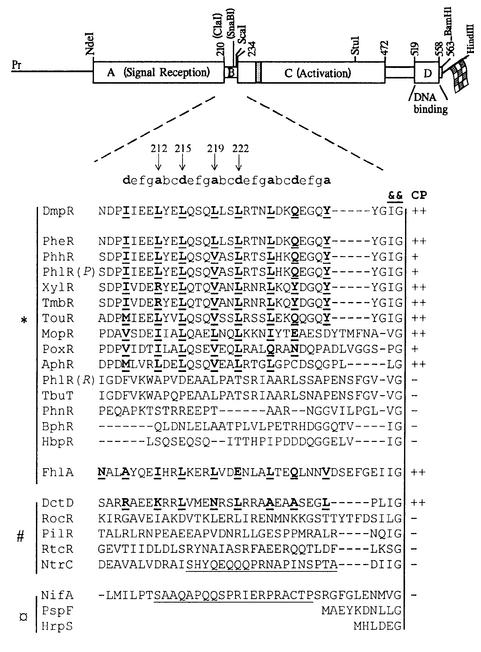
The σ54-dependent activator DmpR regulates transcription of the dmp operon of Pseudomonas CF600, which encodes enzymes for catabolism of (methyl)phenols (Shingler et al., 1993). σ54-dependent regulators control transcription from –24/–12 promoters through binding to upstream activator sites and are brought into contact with σ54–RNA polymerase to provide ATP-dependent initiation of transcription (Kustu et al., 1991). Regulators of this family typically have a domain structure (see Figure 1) in which the highly conserved central C-domain is thought to determine the essential transcriptional activation properties of the regulators, namely ATP hydrolysis (Weiss et al., 1991; Austin and Dixon, 1992) and interaction with σ54–RNA polymerase (Lee and Hoover, 1995; Wang et al., 1997). The N-terminal A-domains define different subgroups and, where present, are involved in controlling the activity of the regulator in response to environmental signals (Shingler, 1996). For the archetypal members NtrC and DctD, signal perception is mediated through sensory protein kinases resulting in phosphorylation of the A-domain of the cognate regulator (Ninfa et al., 1987; Keener and Kustu, 1988; Huala et al., 1992; Klose et al., 1993; Gu et al., 1994). In the case of NifA and PspF (which lacks an A-domain), signal-responsive control is mediated through regulatory protein–protein interactions (Austin et al., 1994; Berger et al., 1994; Dworkin et al., 2000). DmpR, however, belongs to the distinct mechanistic subgroup that directly senses and responds to small effector ligands. This group comprises many aromatic-responsive regulators (see Figure 1) typified by the (methyl)phenol-responsive DmpR (Shingler and Moore, 1994; Shingler and Pavel, 1995) and xylene/toluene-responsive XylR (Delgado and Ramos, 1994), but also includes the mechanistically related formate-responsive FhlA (Hopper et al., 1994).
Fig. 1. Coils analysis of the linker region of DmpR and other σ54-dependent transcriptional regulators. Above, schematic representation of dmpR indicating the location of restriction sites used in generating DmpR derivatives. The domain structure of DmpR, discussed in the text, is superimposed on the restriction map. The shaded box indicates the location of the ATP-binding motif of the family (Morett and Segovia, 1993) and the flag represents a C-terminal fusion of an eight amino acid epitope tag. Below, alignment of the linker region of selected members of the σ54-dependent family of transcriptional activators. Alignment was made relative to two highly conserved residues, a hydrophobic residue followed by glycine (indicated by &&), which mark the start of the C-domain (Jaspers et al., 2000). The a- and d-positions within heptad (a-b-c-d-e-f-g) repeats of proteins predicted to form a coiled coil by the Coils program (Lupas et al., 1991) are shown in bold and underlined. Arrows indicate residues of DmpR targeted in specific mutants used in this study. ‘CP’ indicates the propensity prediction to form a coiled coil of >90% (++), >50% (+) or <50% (–). The linker regions of the different family members are grouped according to known or suspected activation mechanisms with * indicating small-ligand responsive regulators, # indicating phosphorylation-responsive regulators, and ¤ indicating those lacking an A-domain and/or controlled by protein–protein interactions. Underlined sequence indicates the Q-linker regions of NtrC and NifA referred to in the text. Sequences are: AphR from Comamonas testoseroni TA441 (X68033; Arai et al., 1998); BphR encoded on plasmid pNL1 from Sphingomonas aromaticivorans F199 (AF079317; Romine et al., 1999); DctD from Rhizobium meliloti (P13632; Watson, 1990); DmpR encoded on plasmid pVI150 from Pseudomonas sp. strain CF600 (A47078; Shingler et al., 1993); FhlA from E.coli K-12 (P19323; Schlensog and Bock, 1990); HbpR from Pseudomonas azelaica HBP1 (U73900; Jaspers et al., 2000); HrpS from Pseudomonas syringae (P26316; Xiao et al., 1994); MopR from Acinetobacter calcoaceticus NCIB8250 (Z69251; Schirmer et al., 1997); NifA from Klebsiella pneumoniae (P03027; Arnold et al., 1988); NtrC from K.pneumoniae (P03029; Drummond et al., 1983); PheR from Pseudomonas putida BH (D63814; M.Takeo, unpublished); PhhR from P.putida P35X (X79599; Ng et al., 1995); PhlR encoded on plasmid pPGH1 from P.putida H (Müller et al., 1996) indicated in the figure as PhlR(P); PhlR from Ralstonia eutropha JMP134 (AF065891; Ayoubi and Harker, 1998) indicated in the figure as PhlR(R); PhnR from Burkolderia sp. strain RP007 (AF061751; Laurie and Lloyd-Jones, 1999); PilR from Pseudomonas aeruginosa PA01 (Q00934; Ishimoto and Lory, 1992); PoxR from R.eutropha E2 (GAF026065; Hino et al., 1998); PspF from E.coli K-12 (P37344; Jovanovic et al., 1997); RocR from Bacillus subtilis (P38022; Calogero et al., 1994); RtcR from E.coli K-12 (P38035; Blattner et al., 1997); TbuT from Ralstonia pickettii PK01 (U72645; Byrne and Olsen, 1996); TmbR from P.putida TMB (U41301; Favaro et al., 1996); TouR from P.putida (AJ005663; Bertoni et al., 1998); and XylR encoded on plasmid pWW0 from P.putida mt-2 (M10143; Inouye et al., 1985).
The various substrates of the dmp pathway, and some structural analogues, can serve as aromatic effectors of DmpR (Shingler and Moore, 1994; Shingler and Pavel, 1995). We have previously shown that the multiple effectors of DmpR mediate their action through a single common binding site on the A-domain (O’Neill et al., 1998, 1999). During productive activation by aromatic effectors, the binding of aromatic compounds alleviates inhibitory interactions between the A- and C-domains, with concomitant release of its catalytic ATPase activity and transcriptional activating property (Fernández et al., 1995; Pérez-Martín and de Lorenzo, 1995; Shingler and Pavel, 1995; Ng et al., 1996). Hence the A-domain of DmpR mediates two distinct properties: binding of effectors and repression of the C-domain-mediated activities; it can perform both activities autonomously in vitro as an independently expressed domain. However, release of its inter-domain repression of the C-domain in response to binding of effectors is completely dependent on physical continuity, which is normally provided by the short B-linker (O’Neill et al., 1998). Based on mutational evidence that various insertions in the Q-rich B-linker of both NtrC and NifA do not affect their function, the B-linker has generally been considered to serve simply as a flexible linker (Wootton and Drummond, 1989). However, proline substitutions in the B-linkers of both DmpR and its close relative XylR result in semi-constitutive regulators that can promote some degree of transcription in the absence of their activating signals (Fernández et al., 1995; Shingler and Pavel, 1995). Residues that link sensory and output domains of many regulators commonly serve more active functions than that of a simple tether (Spronk et al., 1999). The dissimilar effects of B-linker insertions and substitutions suggest functional differences between the B-linkers of different σ54-dependent activators and point towards an active role for the DmpR and XylR B-linkers. These observations, together with the finding that the presence of the linker region on the DmpR A-domain peptide appears to impart some structural constraint on its ability to bind phenol (O’Neill et al., 1998), prompted us to inspect this region more closely. This examination identified heptad repeats containing regularly spaced hydrophobic residues within the B-linker (Figure 1). Heptad repeats (a-b-c-d-e-f-g), in which the a- and d-positions are hydrophobic, are the signature of the α-helical coiled coils. There are ∼3.5 residues per turn of the α-helix, so the a- and d- hydrophobic residues form stripes on the same side of the helix. Protein subunit interactions are driven by the hydrophobic stripes buried in an interface formed by at least two α-helical chains. These interactions may extend throughout the length of a protein or be very short, involving as few as three or four heptad repeats, as is the case for many transcription factors (for review see Lupas, 1996). The mutational analysis of DmpR reported here is consistent with the B-linker forming a self-associated coiled-coil structure, and demonstrates that the structure of the B-linker is critical for the ability of DmpR to respond to the aromatic-binding signal. The properties of B-linker mutants and truncated DmpR derivatives demonstrate that the level of ATPase activity can be uncoupled from both the level of transcriptional activation and control by aromatic effectors. These results cannot be explained by the existing model for control of σ54-dependent regulators by aromatic effectors. Based on the novel properties of the B-linker DmpR mutants, we propose an alternative model in which alleviation of B-linker repression of ATP hydrolysis and A-domain repression of transcriptional activation are distinct events that are coordinated by allosteric changes induced by binding of aromatic effectors.
Results and discussion
The B-linker of DmpR contains coiled-coil signature heptad repeats
Analysis of the B-linker region of σ54-dependent transcriptional regulators using the Coils program (Lupas et al., 1991) revealed two potential classes of this type of regulator (Figure 1). The B-linkers of DmpR, XylR and some but not all ligand-responsive members of the family are predicted to form short α-helical coiled coils, as is the phosphorylation-responsive DctD. Significantly, the B-linkers of NtrC and NifA, which are tolerant of amino acid insertions in this region (Wootton and Drummond, 1989), are not predicted to form a coiled-coil structure. To elucidate the function of the B-linker region and to dissect the potential role of the identified heptad repeats in control of DmpR by aromatic effectors, we took a mutagenesis approach. This approach used a derivative of dmpR in which unique ClaI and SnaBI sites had been introduced (see Figure 1). Substitution of the ClaI–SnaBI fragment with specifically designed oligonucleotides allows the 12 core residues of the B-linker (211-ELYELQSQLLSL-222, see Figure 1), which span the centre of the heptad repeats, to be replaced with any desired sequence of amino acids. The resulting DmpR derivatives all contain a C-terminal fusion of an eight amino acid ‘Flag tag’. This tag does not alter the magnitude or specificity of the response of DmpR to aromatic effectors in vivo, and allows affinity purification of the proteins for in vitro analysis of ATPase and aromatic effector binding properties (Shingler and Pavel, 1995; O’Neill et al., 1998).
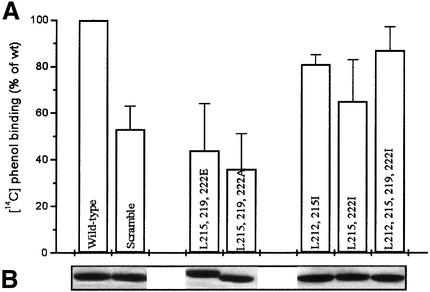
As outlined in the Introduction, the B-linker of DmpR is involved in signal transduction upon aromatic effector binding. To interpret the phenotypes of linker mutants and dissect the structural contribution of this region, it was important, therefore, that the mutant derivatives could still bind aromatic effectors. Hence, the [14C]phenol-binding capacities of all the DmpR-Flag B-linker mutants, including one in which the order of the B-linker residues was ‘scrambled’ and five with specific amino acid substitutions, were determined at a phenol concentration of 16 µM, corresponding to the Kd for phenol binding of wild-type DmpR-Flag. As shown in Figure 2, all derivatives retained >35% of the ability of the wild-type protein to bind the effector at this low concentration. We have previously shown, using an A-domain mutant (DmpR-E135K-Flag), that this level of binding is more than adequate for wild-type levels of in vivo transcriptional activation when assessed with effector concentrations of >1 mM (O’Neill et al., 1999). As will be shown below, the most affected derivative (L215,219,222A, Figure 2) had essentially wild-type ability to respond to aromatic effectors in vivo. These results allow us to attribute defects in the ATPase and transcription-promoting properties of DmpR mutants to the effects of specific amino acid substitutions on B-linker function.
Fig. 2. [14C]phenol binding of Flag-tagged DmpR derivatives. (A) [14C]phenol binding of DmpR-Flag derivatives was measured at a phenol concentration of 16 µM (the Kd for wild-type DmpR-Flag). The value for DmpR-Flag was set at 100% and the data represent the average of triplicate determinations. (B) Coomassie Blue stain of proteins separated by 11% SDS–PAGE after release from 3 µl of anti-Flag beads treated with the indicated extracts, which were used for both [14C]phenol binding and ATPase analysis.
Effector activation requires ordered sequence of heptad repeats
To determine whether the ordered array of amino acid residues within the heptad repeats is critical for effector activation, we generated a mutant designated DmpR-scramble-Flag, which would be expected to generate a loss-of-function phenotype. In this mutant, the central 12 residues of the motif are shuffled to disrupt the ordered sequence, but retain the wild-type amino acid composition and thus innate flexibility and overall hydrophobicity. The ability of DmpR-scramble-Flag to respond to aromatics was compared with that of DmpR-Flag using a previously constructed luciferase reporter system in which the DmpR-controlled Po promoter is fused to luxAB on the host P. putida chromosome. Plasmids expressing DmpR derivatives complete the experimental system. In vivo transcription in response to the presence of the best (2-methylphenol) and poorest (4-methylphenol) natural effectors of DmpR were measured. The DmpR-Flag and DmpR-scramble-Flag proteins are expressed at the same level (Figure 3A) but the DmpR-scramble-Flag derivative is totally incapable of responding to the aromatic effectors in vivo (compare histograms in Figure 3B and C). A second linker scramble mutant, in which the residues in the d-positions were left intact (LELSLQLQSEYL), also showed severe defects in response to aromatic effectors, with <10% wild-type levels of response to 2-methylphenol and an inability to respond to 4-methylphenol (data not shown). We conclude that the ordered sequence of the amino acids within the B-linker is critical for responsiveness of DmpR to aromatic effectors.
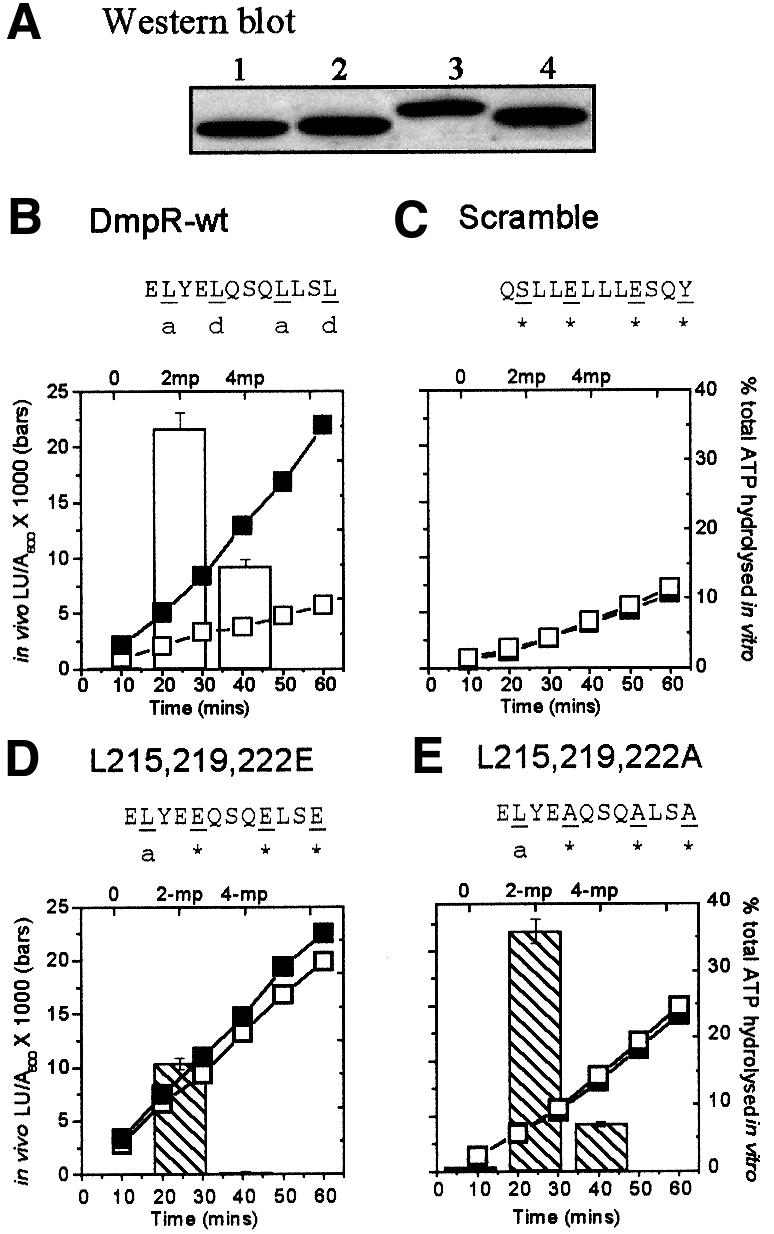
Fig. 3. Comparison of in vivo and in vitro activities of DmpR-Flag derivatives. (A) Western analysis of expression levels of DmpR-Flag (lane 1), DmpR-scramble-Flag (lane 2), DmpR-L215,219,222E-Flag (lane 3) and DmpR-L215,219,222A-Flag (lane 4) in 30 µg of total protein derived from cultures used to determine in vivo transcriptional responses. (B–E) Histograms show the in vivo luciferase transcriptional response of P.putida KT2440::Po-luxAB harbouring plasmids expressing DmpR-Flag (B), DmpR-scramble-Flag (C), DmpR-L215,219,222E-Flag (D) or DmpR-L215,219,222A-Flag (E) measured in the absence or presence of 2 mM 2-methylphenol (2-mp) or 4-methylphenol (4-mp). Values are the averages of triplicate determinations from each of two independent experiments. Line graphs show the level of in vitro ATP hydrolysis mediated by 1 µl of bead-bound Flag-tagged protein, in the absence (open symbols) and presence (closed symbols) of 1 mM 2-methylphenol. Data are the average of duplicate experiments and expressed as the percentage of total ATP (180 nM) hydrolysed.
To test whether the scrambling of the B-linker also results in failure to produce a productive rearrangement that releases the ATPase activity of DmpR, we tested the ability of DmpR-scramble-Flag to hydrolyse ATP in vitro in response to 2-methylphenol. The results, shown in Figure 3C (line graph), demonstrate that DmpR-scramble-Flag is not stimulated by effectors to hydrolyse ATP. The B-linker scramble mutation did not abolish the background level of ATP hydrolysis observed with wild-type DmpR-Flag in the absence of effectors (Figure 3B). This background ATP hydrolysis by DmpR is an innate and specific property of the protein, since introduction of a single amino acid substitution in the ATP binding site (DmpR-G268S-Flag) abolishes even basal ATPase activity (O’Neill et al., 1999). As DmpR-scramble-Flag did not respond to effectors and behaved like DmpR-Flag in the absence of effectors in terms of both its ATPase activity and its inability to promote transcription, it appears that the disordered linker amino acid sequence of DmpR-scramble-Flag locks DmpR in an inactive conformation that cannot respond to the effector-binding signal.
The B-linker of DmpR contains five leucine residues located at the a- and d-positions within the heptad repeats, with those at positions 215, 219 and 222 marking the centre of the whole motif (Figure 1). The hydrophobicity of the a- and d-position residues underlies the coiled-coil structure, and substitutions of these residues by amino acids with different properties would be expected to have differential effects. The Coils program (Lupas et al., 1991) predicts that substitutions of L215, L219 and L222 by the less hydrophobic amino acid alanine would be tolerated since these substitutions only reduce the propensity prediction from 99.8 to 79.0%. Substitutions by the negatively charged glutamic acid, on the other hand, give a much lower predicted propensity value, 15.1%, and thus might be expected to impart at least a partial loss-of-function phenotype. To test these predictions, DmpR-L215,219,222A-Flag and DmpR-L215,219,222E-Flag were generated and subjected to the assays and controls described above for DmpR-Flag and DmpR-scramble-Flag. Both proteins were present at wild-type levels in cells (Figure 3A). However, while DmpR-L215,219,222A-Flag had wild-type ability to promote transcription in response to 2-methylphenol and had only a slightly impaired response to 4-methylphenol, DmpR-L215,219,222E-Flag was much more severely affected (see histograms in Figure 3D and E). These results are consistent with the idea that the B-linker forms a coiled coil that is involved in control of DmpR by aromatic effectors.
The in vitro ATPase activity of DmpR-L215, 219,222E-Flag and DmpR-L215,219,222A-Flag was not repressed in the absence of effectors (Figure 3D and E, line graphs), so substitutions within the B-linker of DmpR can uncouple aromatic effector control of ATPase activity without loss of aromatic effector control of transcription. As expanded upon below, these findings have repercussions for the prevalent model for aromatic effector control of the XylR/DmpR family of transcriptional activators.
Isoleucine substitutions within the B-linker affect response to aromatic effectors
The results described above show the importance of the regular spacing and hydrophobic nature of residues in the a- and d-positions in the heptad repeats. However, it should be noted that analysis of the periodicity of hydrophobic residues cannot distinguish very short coiled- coil structures (such as that predicted for DmpR) from short amphipathic helices. Moreover, the phenotypes of DmpR-L215,219,222A-Flag and DmpR-L215,219,222E-Flag would also be compatible with the predicted effects of alanine and glutamic acid substitutions on a hydrophobic interaction between two amphipathic helices. One major property that distinguishes coiled coils from other amphipathic helices is their distinctive ‘knobs-into-holes’ packaging interaction. Physical constraints imposed by the side chains of residues at the a- and d-positions dictate the number and orientation of the α-helices that can be accommodated (Lovejoy et al., 1993; Harbury et al., 1994). Leucine and isoleucine differ very little in their hydrophobicity but the side-chain packing preference of these amino acids in the a- and/or d-positions lead to dramatic differences in the resulting coiled-coil interactions in peptides. Peptides carrying the four possible combinations of leucine and isoleucine at the a- and d-positions differentially form coiled coils with two, three, four and six chains (Harbury et al., 1993). We reasoned, therefore, that leucine-to-isoleucine substitutions would probably affect a coiled-coil structure but leave a hydrophobic amphipathic helix interaction essentially intact. Hence, we generated a series of derivatives in which the a- and/or d-leucines of the core 12 residues of the B-linker were substituted for isoleucine. All the leucine-to-isoleucine substitution mutants were expressed at wild-type levels in cells (Figure 4A). As shown in Figure 4, DmpR-L212,219I-Flag (a-position substitutions; dimer in peptides) and DmpR-L215,222I-Flag (d-position substitutions; tetramer in peptides) had impaired ability to promote transcription in response to aromatic effectors, while DmpR-L212,215,219,222I-Flag (a- and d-position substitutions; trimer in peptides) was only marginally affected in this respect. The location of the leucine-to-isoleucine substitutions had a graded effect on the control of ATPase activity by aromatic effectors, ranging from no effect in the case of a-position substitutions (Figure 4B), through partial uncoupling in the case of d-position substitutions (Figure 4C) to complete uncoupling with isoleucine substitutions in both a- and d-positions (Figure 4D). These results strongly favour the interpretation that the heptad repeats of the B-linker of DmpR specify a coiled-coil structure rather than an amphipathic helix. They also demonstrate that ATPase activity is uncoupled from aromatic-effector control and from the resulting level of transcriptional activation (Figure 4D).
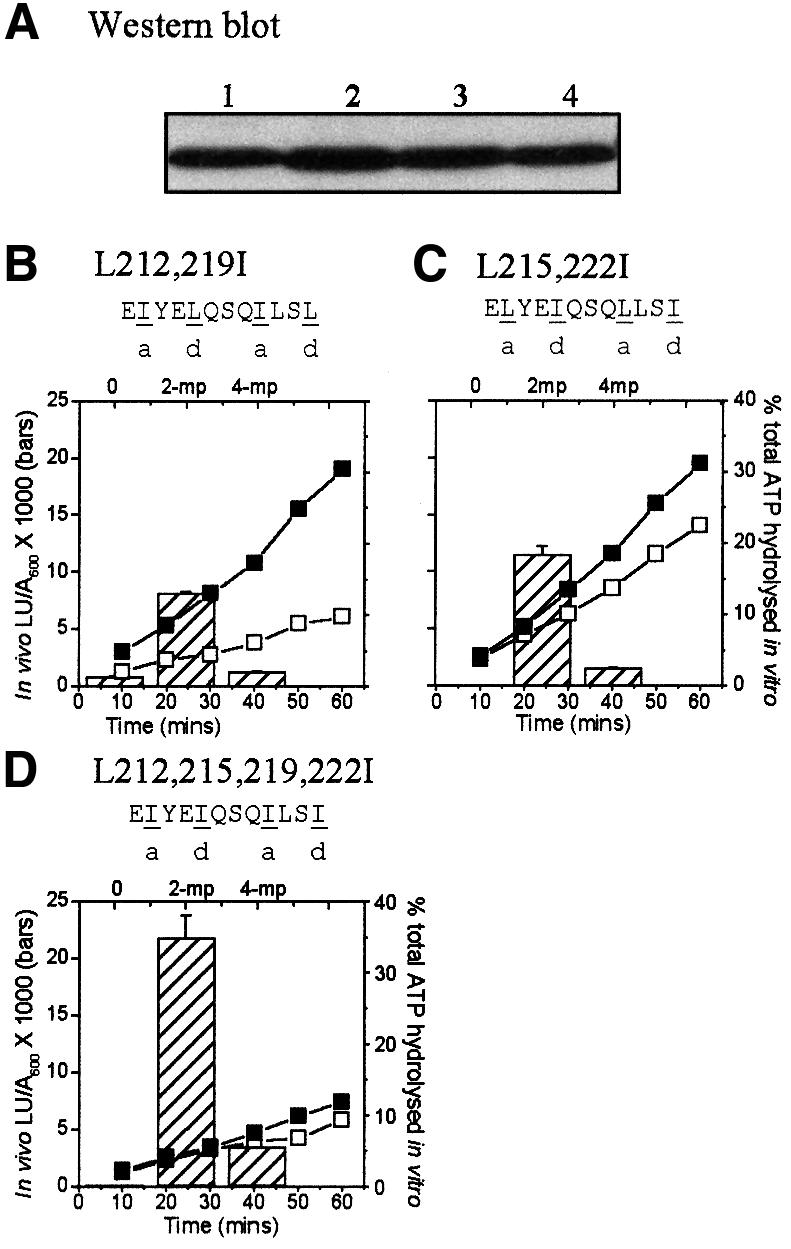
Fig. 4. In vivo and in vitro responses of DmpR-Flag derivatives with leucine to isoleucine substitutions within the linker. (A) Western blot and (B–D) in vivo transcriptional responses (histograms) and in vitro ATPase activities (line graphs) are as in Figure 3. DmpR-Flag (lane A1), DmpR-L212,219I-Flag [lane A2 and (B)], DmpR-L215,222I-Flag [lane A3 and (C)] and DmpR-L212,215,219,222I-Flag [lane A4 and (D)]. The predicted coiled-coil propensity values for these mutants are: DmpR-L212,219I-Flag, 78%; DmpR-L215,222I-Flag, 41%; and DmpR-L212,215,219,222I-Flag, 21%.
While deductions from peptide analysis cannot always be translated to behaviour within intact proteins (see Lupas, 1996), it is interesting to note that di-, tetra- and hexameric coiled coils are observed in peptides containing Leu in both a- and d-positions, as in the B-linker of wild-type DmpR (Harbury et al., 1993). The data above suggest, though this is highly speculative, that stimulated ATPase activity might be compatible with dimeric/tetrameric arrangement of the B-linker, while efficient transcriptional activation may be compatible with trimeric/hexameric arrangements.
Effector-stimulated ATPase activity is not required for efficient transcriptional activation in vivo
A striking feature of the mutant analysis described above is the ability of DmpR-L212,215,219,222I-Flag to promote efficient transcriptional activation in vivo in the absence of effector-stimulated ATPase activity. Irrespective of the presence of aromatic effectors, this derivative had only basal ATPase levels equivalent to that of wild-type DmpR-Flag in the absence of effectors (compare Figures 3B and 4D). This suggests either that the conformation of DmpR-L212,215,219,222I-Flag renders it capable of promoting efficient transcription with only basal ATPase activity or that it may be able to promote transcription in the absence of ATP binding and/or hydrolysis. To distinguish between the two possibilities, we used three single amino acid substitutions that are critical for ATP binding or hydrolysis. DmpR with a G268S substitution (within the ATP-binding motif) is unable to bind ATP, while DmpR with an E335L or L356P substitution can bind ATP but cannot hydrolyse it. All these single amino acid substitutions completely abolish ATP hydrolysis by A-domain-deleted derivatives of DmpR (Ng et al., 1996; P.Wikström, E.O’Neill, L.C.Ng and V.Shingler, in preparation). These single amino acid substitutions were combined with the leucine-to-isoleucine substitutions in DmpR-L212,215,219,222I-Flag. As shown in Figure 5, none of the resulting derivatives promoted transcription in vivo. Taken together, these results suggest that the leucine-to-isoleucine substitutions of DmpR-L212,215,219,222I-Flag relieve some structural constraint that allows full transcriptional levels in vivo with only basal ATP activity of DmpR.
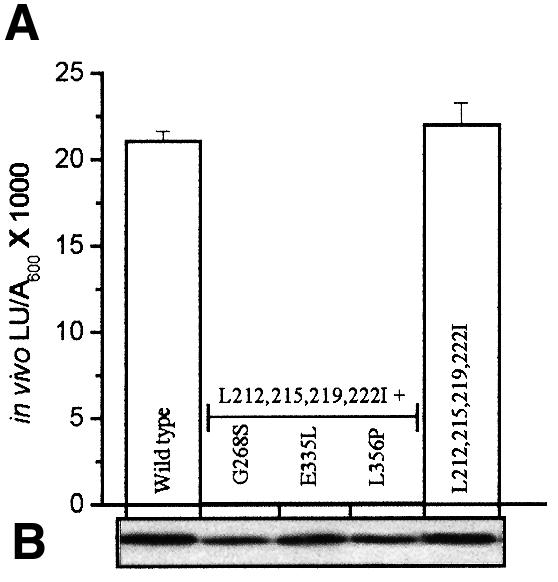
Fig. 5. In vivo transcriptional responses of DmpR-Flag derivatives with ATP binding and/or hydrolysis defects and leucine-to-isoleucine substitutions within the linker. (A) In vivo transcriptional responses of the indicated DmpR-Flag derivatives in response to 2-methylphenol and (B) western analysis of expression levels in the cells used, as described in the legend to Figure 3.
B-linker controls ATPase activity of DmpR
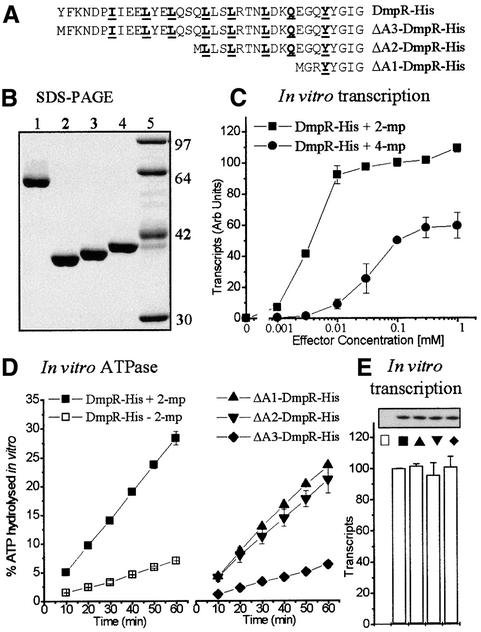
In addition to uncoupling ATP hydrolysis from proficiency in transcriptional activation, the B-linker mutants also uncouple ATPase activity from aromatic effector control. This begs the questions of what determines the level of ATPase activity in DmpR and how the ATPase activity is normally controlled in response to aromatic effectors. To address these questions, we generated and purified a series of C-terminal His-tagged DmpR derivatives (Figure 6A and B). The epitope tag of DmpR-His, like that of DmpR-Flag, did not alter the regulatory properties of DmpR in vivo (data not shown). Moreover, DmpR-His mediated in vitro transcription in response to increasing concentrations of aromatic effectors (Figure 6C) faithfully reproduced both the dose–response and maximal relative levels with which 2- and 4-methylphenol promote transcription by DmpR in vivo (O’Neill et al., 1999). As with DmpR-Flag bound to beads, the basal level of in vitro ATP hydrolysis by DmpR-His in the presence of its specific DNA-binding sites and RNA polymerase was ∼20% of fully effector-activated levels (Figure 6D).
Fig. 6. Purification and in vitro activities of DmpR-His derivatives. (A) The linker sequence and (B) Coomassie Blue stain of proteins separated by 11% SDS–PAGE: lane 1, DmpR-His; lane 2, ΔA1-DmpR-His; lane 3, ΔA2-DmpR-His; lane 4, ΔA3-DmpR-His; lane 5, standards. (C) In vitro transcription mediated by DmpR-His in response to increasing concentrations of the effectors 2-methylphenol (2-mp) and 4-methylphenol (4-mp). (D and E) In vitro ATPase activity (D) and transcription (E) mediated by the indicated derivatives in a coupled assay in the absence or presence of 0.5 mM 2-methylphenol. The results are the average of duplicate determinations.
The uncoupling of ATPase activity from transcription-promoting ability observed with the linker mutants suggests that in the native protein the B-linker keeps the ATPase activity in check and that allosteric changes upon effector binding relieve the inhibitory effect of the linker. If this were the case, then deletion of the A-domain and B-linker of DmpR should result in a derivative with full ATPase activity. Deletion of just the A-domain, on the other hand, might be expected to leave B-linker-mediated repression of the ATPase activity intact and therefore result in a derivative with just basal ATPase levels. The previous finding that deletion of the A-domain and the N-terminal half of the B-linker results in a derivative, ΔA2-DmpR, which has constitutive ATPase activity and transcriptional activation properties equivalent to that of fully activated wild-type protein (Shingler and Pavel, 1995; O’Neill et al., 1999) is consistent with this idea. However, to test the hypothesis rigorously, we generated, in addition to ΔA2-DmpR-His, A-domain-truncated DmpR derivatives that differed in possession of the B-linker: ΔA1-DmpR-His completely lacks both the A-domain and B-linker while ΔA3-DmpR-His lacks just the A-domain (Figure 6A and B). The ability of these derivatives to hydrolyse ATP and promote transcription in vitro was determined from the same reaction mixtures and compared with that of DmpR-His in the presence and absence of 2-methylphenol (Figure 6D and E). The data show that the predictions hold true since the B-linker-containing derivative (ΔA3) mediated only basal ATPase activity, while the B-linker-deleted (ΔA1 and ΔA2) derivatives both mediated high ATPase activities similar to that of effector-activated wild-type DmpR-His (Figure 6D). Irrespective of the level of ATPase activity, ΔA1-, ΔA2- and ΔA3-DmpR-His were equally proficient at promoting transcription in vitro to a level equivalent to that of effector-activated DmpR-His (Figure 6E). Thus, as with the B-linker mutants, ATPase activity and the ability to promote transcription can be uncoupled.
Two plausible mechanisms, which are compatible with both the deletion analysis and the B-linker mutant data, can explain the B-linker-mediated control of the ATPase activity of DmpR. In both cases, allosteric changes upon A-domain binding of effectors alleviate B-linker-mediated constraints on the ATP-hydrolysing C-domain. In the first case, and by analogy with the mechanism of the metal-binding ArsA-dependent ATPase pump (Li and Rosen, 2000), relief of B-linker-mediated constraints may result in optimal contacts between two or more ATP-binding domains and thus facilitate catalysis. Alternatively, allosteric changes may modify the ATP-binding affinities. In either case, effector binding would coordinately result in alleviation of both B-linker repression of the catalytic ATPase activity and A-domain inhibition of interaction with σ54–RNA polymerase.
Integrated model for effector activation of DmpR
The prevailing model for aromatic effector control of the DmpR/XylR class of regulators has been that the A-domain controls transcription-promoting activity simply by controlling the level of essential ATPase activity. This model was primarily based on the findings that aromatic effectors control ATP hydrolysis by DmpR in vitro (Shingler and Pavel, 1995) and that deletion of the A-domains of DmpR and XylR mimics the state activated by aromatic effectors (Fernández et al., 1995; Shingler and Pavel, 1995). However, the observation that some phenolic compounds can be bound by DmpR and elicit an ATPase activity but do not promote transcriptional activation is difficult to explain using this simple model (O’Neill et al., 1999). Here we demonstrate uncoupling of high aromatic effector-induced levels of ATPase activity from transcription-promoting ability of DmpR in the linker mutants (e.g. DmpR-L212,215,219,222I-Flag; Figure 4D) and with truncation derivatives that differentially possess the B-linker residues (Figure 6D and E). These results provide sufficient evidence to refute the previous model. Binding of aromatic effectors via the A-domain undoubtedly does alleviate repression of the ATPase activity of DmpR. However, since possession of the B-linker results in basal ATPase activity while its deletion results in high ATPase activity (Figure 6D), the data strongly suggest that the B-linker controls this event. Furthermore, since the levels of transcription mediated by derivatives lacking the A-domain are indistinguishable irrespective of basal or high ATPase activity (Figure 6E), the primary regulatory role of the A-domain is likely to be control of transcriptional activation by hindering productive interaction with the σ54-transcriptional apparatus in the absence of effectors. Although stimulation of the ATPase and transcription-promoting activities by effectors can be genetically uncoupled, control of these two events is co-ordinated in the wild-type protein (Figure 6C and D). Taken together, the data support a model in which allosteric changes upon effector binding to the A-domain coordinately alleviate both B-linker repression of the catalytic ATPase activity and A-domain inhibition of interaction with σ54–RNA polymerase.
The analysis of DmpR-L212,215,219,222I-Flag and ΔA1-DmpR-His demonstrates that, in the absence of other constraints, the basal ATPase activity of DmpR is sufficient for full transcription activation (Figures 4D and 6E). This raises the question of the role, if any, of effector-stimulated ATPase activity. One possibility is that it helps to counteract inhibitory A/C-domain interactions that silence DmpR in the absence of aromatic effectors and are presumably relatively positioned by the B-linker. In this respect, it is interesting that, for the mechanistically related FhlA regulator, effector (formate)-dependent transcription in vitro is only observed at low ATP concentrations, while at high ATP concentrations transcription becomes completely independent of effector (Hopper et al., 1996).
The B-linker mutant analysis demonstrates that the heptad repeats of this region are critical for the response of DmpR to its aromatic effector-binding signal. Whether short sequences of heptad repeats take up a coiled-coil conformation or not can be context dependent, being dictated by binding of a protein to DNA or the context provided by the flanking regions (Lupas, 1996). Scrambling of the B-linker residues totally abolishes the ability to respond to effectors both in terms of high ATPase activity and ability to promote transcription (Figure 3C), while concomitant deletion of both the A-domain and the B-linker results in constitutive high ATPase activity (Figure 6D). These results suggest either that a structured B-linker is required for relief of repression of ATPase activity upon effector activation or that, in the scrambled mutant, the A-domain is aligned and represses the C-domain in a different manner than in the wild-type protein.
Concluding remarks
The mutant and deletion analysis presented here unequivocally demonstrates that the B-linker possesses a structure that is actively involved in and critical to the response of DmpR to its aromatic effector-binding signal. The observation of similar signature heptad repeats within both effector-responsive and phosphorylation-responsive σ54-dependent regulators suggests that an active role of the B-linkers is probably a common mechanistic feature of a whole subgroup of the family. It is plausible that possession of the heptad repeats may underlie significant differences in the outcome of deletions of the N-terminal regulatory domains, such as those observed between the phosphorylation-responsive regulators NtrC and DctD. In this respect, it is interesting to note that DctD possesses heptad repeats and, similar to DmpR, N-terminal truncation of the regulatory domain results in a signal-independent constitutively active derivative (Lee et al., 1994). Conversely, N-terminal truncation of NtrC, which does not possess heptad repeats in the B-linker, results in a derivative incapable of promoting transcriptional activation (Porter et al., 1995).
Materials and methods
DNA manipulations and general procedures
Construction of broad-host-range plasmids expressing dmpR (pVI401) or dmpR-Flag (pVI455) with a fusion of an eight amino acid epitope, Flag tag (Hopp et al., 1988), from the native promoter for dmpR has previously been described (Shingler and Moore, 1994; Shingler and Pavel, 1995). A similar plasmid, pVI545, contains a ClaI site overlapping codon 210 of dmpR-Flag (which results in an E210D substitution) and a silent SnaBI site overlapping codons 221 and 222 (see Figure 1; Skärfstad et al., 2000). DmpR-Flag linker mutants were generated by replacing the ClaI–SnaBI fragment of pVI545 with synthetic oligonucleotides encoding the desired sequence and that regenerated a glutamic acid codon for E210. This strategy thus resulted in plasmids expressing the derivatives from the native dmpR promoter. Replacement of the NdeI–ScaI fragment of pVI401-derived plasmids carrying ATP-binding or hydrolysis mutants of DmpR by that of dmpR-L212,215,219,222I generated DmpR derivatives with the L212,215,219,222I linker mutation in the context of different ATP defects. T7 expression plasmids for DmpR-Flag linker mutations were constructed by cloning NdeI–HindIII fragments spanning the entire regulator and Flag tag into pET3H as previously described for DmpR-Flag (pVI456; Shingler and Pavel, 1995). For expression of His-tagged proteins, DmpR-His, ΔA1-DmpR-His and ΔA2-DmpR-His, analogous T7 expression plasmids were constructed by replacing the BamHI–HindIII Flag tag fragment (Shingler and Pavel, 1995) with oligonucleotides that introduced a serine codon and six histidine codons followed by a stop codon. For ΔA3-DmpR-His, a T7 expression plasmid was constructed using the same PCR-based strategy as described for ΔA1-DmpR (Shingler and Pavel, 1995). The DNA sequence integrity of all oligonucleotides and PCR-generated DNA was confirmed. Plasmids expressing DmpR-Flag derivatives were introduced into the luciferase reporter P.putida KT2440:: Po-luxAB and assayed as previously described (Sze et al., 1996). Protein levels of DmpR-Flag derivatives were determined by western blot analysis using an M2 mouse monoclonal antibody against Flag (Sigma) as previously described (Shingler and Pavel, 1995).
Purified protein
Core RNA polymerase was purchased from Epicentre Technology; Escherichia coli σ54 and IHF (integration host factor) were purified essentially as previously described (Hunt and Magasanik, 1985; Vorgias and Wilson, 1991). T7 promoter-driven expression of DmpR derivatives and crude extract preparation were as previously described (Shingler and Pavel, 1995). DmpR-Flag derivatives were affinity purified using Flag-M2 affinity gel (Sigma) as described previously (Shingler and Pavel, 1995). The bead-bound proteins were resuspended as a slurry in ATPase assay buffer [35 mM Tris–acetate pH 7.9, 5 mM Mg(OAc)2, 70 mM KAc, 20 mM NH4Ac, 1 mM dithiothreitol (DTT)] and kept on ice until their use in ATPase or [14C]phenol-binding assays. The concentration of bead-bound proteins was determined by SDS–PAGE comparison of a serial dilution of each sample against standards of known concentration in the linear 0.5–3 µg range and quantification by densitometry. His-tagged derivatives were purified using 1 ml nickel chelating columns (Pierce) equilibrated with buffer A (20 mM NaPO4 pH 7.2, 0.5 M NaCl, 5 mM imidazole, 10% glycerol, 0.1% ultrapure Triton X-100). Protein-loaded columns were extensively washed with buffer A containing 100 mM imidazole and the His-tagged DmpR derivatives recovered by stepwise elution using buffer A containing 0.2–1 M imidazole. The most concentrated fractions were clarified through gel filtration Bio-Spin P30 columns (Bio-Rad) equilibrated with storage buffer (20 mM Tris–HCl pH 7.5, 0.5 M NaCl, 1 mM EDTA, 2 mM β-mercaptoethanol, 30% glycerol, 0.25% ultrapure Triton X-100) and stored at –80°C. Protein concentrations were determined by a BCA (bicinchoninic acid) assay (Pierce) with bovine serum albumin (BSA) as a standard. This purification procedure, when used with a DmpR His-tagged derivative with the ATP-binding mutation G268S, yielded a preparation with no detectable ATPase activity (data not shown).
[14C]phenol-binding assays
Flag-tagged proteins were tested for the ability to bind custom-synthesized universally labelled [14C]phenol (5.22 Gbq/mmol; Amersham Pharmacia Biotech). Experiments were performed at a final concentration of 16 µM phenol (the Kd of wild-type DmpR-Flag) using bead-bound proteins, with background levels bound to a ΔA-NifA-Flag derivative subtracted to determine specific binding, as previously described (O’Neill et al., 1998). Values are expressed as a percentage of [14C]phenol bound by wild-type DmpR-Flag.
ATPase assays with Flag-tagged proteins
ATPase assays were performed at 30°C in 1× ATPase assay buffer (see above) and 1 mM 2-methylphenol where indicated, as previously described (Shingler and Pavel, 1995). Reactions (60 µl) contained 1 µl of bead-bound protein (0.3 µg). Assays were initiated by the addition of [γ-32P]ATP (Amersham Pharmacia Biotech) diluted (1:9) with unlabelled ATP and added to a final concentration of 3 mM.
Coupled in vitro transcription and ATPase assays
Plasmid pTE-Po, which carries the binding sites for DmpR and the dmp operon promoter and directs formation of a 311 base RNA (Carmona et al., 2000), was used as the DNA template. Supercoiled plasmid DNA was prepared by CsCl gradients, extensively dialysed and clarified through Micro Bio-Spin P30 columns (Bio-Rad) equilibrated with sterile water. Single-round transcription assays were performed at 37°C by a method adapted from Claverie-Martín and Magasanik (1992). Core enzyme (20 nM) and 80 nM σ54 were mixed in transcription buffer (50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 0.275 mg/ml BSA) containing [γ-32P]ATP (3 mM, diluted 1:9) for 5 min to allow holoenzyme formation. Template (5 nM), IHF (10 nM), His-tagged DmpR derivatives (100 nM) and the indicated concentration of effector were then added to initiate the reaction. For measurement of ATPase hydrolysis, samples were removed at 10 min intervals and treated as described above. For transcripts, after 15 min incubation at 37°C to allow open complex formation, an aliquot of the same mix was placed in a separate tube and a single round of transcription was initiated by adding a mixture of ATP, GTP, CTP (final concentration 0.4 mM each), UTP (final concentration 0.06 mM), [α-32P]UTP (5 µCi at >3000/mmol) and heparin (0.1 mg/ml, to prevent re-initiation). After an additional 10 min at 37°C, the reactions were terminated and analysed on a 7 M urea/4% polyacrylamide sequencing gel; the density of bands was quantified using a Molecular Dynamics PhosphorImager.
Acknowledgments
Acknowledgements
We thank J.Garmendia, V.de Lorenzo and M.Gullberg for fruitful discussion, E.Skärfstad for technical assistance and M.Gullberg for critical reading of the manuscript. This work was supported by grants from the Swedish Research Council for Natural Science, The Swedish Foundation for Strategic Research, and the J.C.Kempe Foundation.
REFERENCES
- Arai H., Akahira,S., Ohishi,T., Maeda,M. and Kudo,T. (1998) Adaptation of Comamonas testosteroni TA441 to utilize phenol: organization and regulation of the genes involved in phenol degradation. Microbiology, 144, 2895–2903. [DOI] [PubMed] [Google Scholar]
- Arnold W., Rump,A., Klipp,W., Priefer,U.B. and Puhler,A. (1988) Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae.J. Mol. Biol., 203, 715–738. [DOI] [PubMed] [Google Scholar]
- Austin S. and Dixon,R. (1992) The prokaryotic enhancer binding protein NTRC has an ATPase activity which is phosphorylation and DNA dependent. EMBO J., 11, 2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Buck,M., Cannon,W., Eydmann,T. and Dixon,R. (1994) Purification and in vitro activities of the native nitrogen fixation control proteins NifA and NifL. J. Bacteriol., 176, 3460–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubi P.J. and Harker,A.R. (1998). Whole-cell kinetics of trichloroethylene degradation by phenol hydroxylase in a Ralstonia eutropha JMP134 derivative. Appl. Environ. Microbiol., 64, 4353–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger D.K., Narberhaus,F. and Kustu,S. (1994) The isolated catalytic domain of NIFA, a bacterial enhancer-binding protein, activates transcription in vitro: activation is inhibited by NIFL. Proc. Natl Acad. Sci. USA, 91, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni G., Martino,M., Galli,E. and Barbieri,P. (1998) Analysis of the gene cluster encoding toluene/o-xylene monooxygenase from Pseudomonas stutzeri OX1. Appl. Environ. Microbiol., 64, 3626–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F.R. et al. (1997) The complete genome sequence of Escherichia coli K-12. Science, 277, 1453–1474. [DOI] [PubMed] [Google Scholar]
- Byrne A.M. and Olsen,R.H. (1996) Cascade regulation of the toluene-3-monooxygenase operon (tbuA1UBVA2C) of Burkholderia pickettii PKO1: role of the tbuA1 promoter (PtbuA1) in the expression of its cognate activator, TbuT. J. Bacteriol., 178, 6327–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero S., Gardan,R., Glaser,P., Schweizer,J., Rapoport,G. and Debarbouille,M. (1994) RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J. Bacteriol., 176, 1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona M., Rodriguez,M.J., Martinez-Costa,O. and de Lorenzo,V. (2000) In vivo and in vitro effects of (p)ppGpp on the σ54 promoter Pu of the TOL plasmid of Pseudomonas putida. J. Bacteriol., 182, 4711–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie-Martín F. and Magasanik,B. (1992) Positive and negative effects of DNA bending on activation of transcription from a distant site. J. Mol. Biol., 227, 996–1008. [DOI] [PubMed] [Google Scholar]
- Delgado A. and Ramos,J.L. (1994) Genetic evidence for activation of the positive transcriptional regulator Xy1R, a member of the NtrC family of regulators, by effector binding. J. Biol. Chem., 269, 8059–8062. [PubMed] [Google Scholar]
- Drummond M., Clements,J., Merrick,M. and Dixon,R. (1983) Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature, 301, 302–307. [DOI] [PubMed] [Google Scholar]
- Dworkin J., Jovanovic,G. and Model,P. (2000) The PspF protein of Eschericia coli is a negative regulator of σ54-dependent transcription. J. Bacteriol., 182, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro R., Bernasconi,C., Passini,N., Bertoni,G., Bestetti,G., Galli,E. and Deho,G. (1996) Organisation of the tmb catabolic operons of Pseudomonas putida TMB and evolutionary relationship with the xyl operons of the TOL plasmid pWW0. Gene, 182, 189–193. [DOI] [PubMed] [Google Scholar]
- Fernández S., de Lorenzo,V. and Pérez-Martín,J. (1995) Activation of the transcriptional regulator XylR of Pseudomonas putida by release of repression between functional domains. Mol. Microbiol., 16, 205–213. [DOI] [PubMed] [Google Scholar]
- Gu B., Lee,J.H., Hoover,T.R., Scholl,D. and Nixon,B.T. (1994) Rhizobium meliloti DctD, a σ54-dependent transcriptional activator, may be negatively controlled by a subdomain in the C-terminal end of its two-component receiver module. Mol. Microbiol., 13, 51–66. [DOI] [PubMed] [Google Scholar]
- Harbury P.B., Zhang,T., Kim,P.S. and Alber,T. (1993) A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science, 262, 1401–1407. [DOI] [PubMed] [Google Scholar]
- Harbury P.B., Kim,P.S. and Alber,T. (1994) Crystal structure of an isoleucine-zipper trimer. Nature, 371, 80–83. [DOI] [PubMed] [Google Scholar]
- Hino S., Watanabe,K. and Takahashi,N. (1998) Phenol hydroxylase cloned from Ralstonia eutropha strain E2 exhibits novel kinetic properties. Microbiology, 144, 1765–1772. [DOI] [PubMed] [Google Scholar]
- Hopp T.P., Prickett,K.S., Price,V.L., Libby,R.T., March,C.J., Cerretti,D.P., Urdal,D.L. and Conlon,P.J. (1988) A short polypeptide marker sequence useful for recombinant protein identification and purification. Biotechnology, 6, 1204–1210. [Google Scholar]
- Hopper S., Babst,M., Schlensog,V., Fischer,H.M., Hennecke,H. and Bock,A. (1994) Regulated expression in vitro of genes coding for formate hydrogenlyase components of Escherichia coli.J. Biol. Chem., 269, 19597–19604. [PubMed] [Google Scholar]
- Hopper S., Korsa,I. and Bock,A. (1996) The nucleotide concentration determines the specificity of in vitro transcription activation by the σ54-dependent activator FhlA. J. Bacteriol. 178, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E., Stigter,J. and Ausubel,F.M. (1992) The central domain of Rhizobium leguminosarum DctD functions independently to activate transcription. J. Bacteriol., 174, 1428–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T.P. and Magasanik, B. (1985) Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc. Natl Acad. Sci. USA, 82, 8453–8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Nakazawa,A. and Nakazawa,T. (1985) Determination of the transcription initiation site and identification of the protein product of the gene xylR for xyl operons on the TOL plasmid. J. Bacteriol., 163, 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto K.S. and Lory,S. (1992) Identification of pilR, which encodes a transcriptional activator of the Pseudomonas aeruginosa pilin gene. J. Bacteriol., 174, 3514–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers M.C., Suske,W.A., Schmid,A., Goslings,D.A., Kohler,H.P. and van der Meer,J.R. (2000) HbpR, a new member of the XylR/DmpR subclass within the NtrC family of bacterial transcriptional activators, regulates expression of 2-hydroxybiphenyl metabolism in Pseudomonas azelaica HBP1. J. Bacteriol., 182, 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic G., Dworkin,J. and Model,P. (1997) Autogenous control of PspF, a constitutively active enhancer-binding protein of Escherichia coli. J. Bacteriol., 179, 5232–7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener J. and Kustu,S. (1988) Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc. Natl Acad. Sci. USA, 85, 4976–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose K.E., Weiss,D.S. and Kustu,S. (1993) Glutamate at the site of phosphorylation of nitrogen-regulatory protein NTRC mimics aspartyl-phosphate and activates the protein. J. Mol. Biol., 232, 67–78. [DOI] [PubMed] [Google Scholar]
- Kustu S., North,A.K. and Weiss,D.S. (1991) Prokaryotic transcriptional enhancers and enhancer binding proteins. Trends Biochem. Sci., 16, 397–402. [DOI] [PubMed] [Google Scholar]
- Laurie A.D. and Lloyd-Jones,G. (1999) The phn genes of Burkholderia sp. strain RP007 constitute a divergent gene cluster for polycyclic aromatic hydrocarbon catabolism. J. Bacteriol., 181, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H. and Hoover,T.R. (1995) Protein crosslinking studies suggest that Rhizobium meliloti C4-dicarboxylic acid transport protein D, a σ54-dependent transcriptional activator, interacts with σ54 and the β subunit of RNA polymerase. Proc. Natl Acad. Sci. USA, 92, 9702–9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Scholl,D., Nixon,B.T. and Hoover,T.R. (1994) Constitutive ATP hydrolysis and transcription activation by a stable truncated form of Rhizobium meliloti DCTD, a σ54-dependent transcriptional activator. J. Biol. Chem., 269, 20401–20409. [PubMed] [Google Scholar]
- Li J. and Rosen, B.P. (2000) The linker peptide of the ArsA ATPase. Mol. Microbiol., 35, 361–367. [DOI] [PubMed] [Google Scholar]
- Lovejoy B., Choe,S., Cascio,D., McRorie,D.K., DeGrado,W.F. and Eisenberg,D. (1993) Crystal structure of a synthetic triple-stranded α-helical bundle. Science, 259, 1288–1293. [DOI] [PubMed] [Google Scholar]
- Lupas A. (1996) Coiled coils: new strutures and new functions. Trends Biochem. Sci., 21, 375–382. [PubMed] [Google Scholar]
- Lupas A., Van Dyke,M. and Stock,J. (1991) Predicting coiled coils from protein sequences. Science, 252, 1162–1164. [DOI] [PubMed] [Google Scholar]
- Morett E. and Segovia,L. (1993) The σ54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J. Bacteriol., 175, 6067–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Petruschka,L., Cuypers,H., Burchhardt,G. and Herrmann,H. (1996) Carbon catabolite repression of phenol degradation in Pseudomonas putida is mediated by the inhibition of the activator protein PhlR. J. Bacteriol., 178, 2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L.C., Poh,C.L. and Shingler,V. (1995) Aromatic effector activation of the NtrC-like transcriptional regulator PhhR limits the catabolic potential of the (methyl)phenol degradative pathway it controls. J. Bacteriol., 177, 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L.C., O’Neill,E. and Shingler,V. (1996) Genetic evidence for inter-domain regulation of the phenol responsive σ54-dependent activator DmpR. J. Biol. Chem., 271, 17281–17286. [DOI] [PubMed] [Google Scholar]
- Ninfa A.J., Reitzer,L.J. and Magasanik,B. (1987) Initiation of transcription at the bacterial glnAp2 promoter by purified E. coli components is facilitated by enhancers. Cell, 50, 1039–1046. [DOI] [PubMed] [Google Scholar]
- O’Neill E., Ng,L.C., Sze,C.C. and Shingler,V. (1998) Aromatic ligand binding and intramolecular signaling of the phenol-responsive σ54-dependent regulator DmpR. Mol. Microbiol., 28, 131–141. [DOI] [PubMed] [Google Scholar]
- O’Neill E., Sze,C.C. and Shingler,V. (1999) Novel effector control through modulation of a preexisting binding site of the aromatic-responsive σ54-dependent regulator DmpR. J. Biol. Chem., 274, 32425–32432. [DOI] [PubMed] [Google Scholar]
- Pérez-Martín J. and de Lorenzo,V. (1995) The amino-terminal domain of the prokaryotic enhancer-binding protein XylR is a specific intramolecular repressor. Proc. Natl Acad. Sci. USA, 92, 9392–9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S.C., North,A.K. and Kustu,S. (1995) Mechanism of transcriptional activation by NtrC. In Silhavy,T. and Hoch,J. (eds), Two-Component Signal Transduction. ASM Press, Washington, DC, pp. 147–158.
- Romine M.F., Stillwell,L.C., Wong,K.K., Thurston,S.J., Sisk,E.C., Sensen,C., Gaasterland,T., Fredrickson,J.K. and Saffer,J.D. (1999) Complete sequence of a 184-kilobase catabolic plasmid from Sphingomonas aromaticivorans F199. J. Bacteriol., 181, 1585–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer F., Ehrt,S. and Hillen,W. (1997) Expression, inducer spectrum, domain structure, and function of MopR, the regulator of phenol degradation in Acinetobacter calcoaceticus NCIB8250. J. Bacteriol., 179, 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlensog V. and Bock,A. (1990) Identification and sequence analysis of the gene encoding the transcriptional activator of the formate hydrogenlyase system of Escherichia coli.Mol. Microbiol., 4, 1319–1327. [DOI] [PubMed] [Google Scholar]
- Shingler V. (1996) Signal sensing by σ54-dependent regulators: derepression as a control mechanism. Mol. Microbiol., 19, 409–416. [DOI] [PubMed] [Google Scholar]
- Shingler V. and Moore,T. (1994) Sensing of aromatic compounds by the DmpR transcriptional activator of phenol-catabolizing Pseudomonas sp. strain CF600. J. Bacteriol., 176, 1555–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingler V. and Pavel,H. (1995) Direct regulation of the ATPase activity of the transcriptional activator DmpR by aromatic compounds. Mol. Microbiol., 17, 505–513. [DOI] [PubMed] [Google Scholar]
- Shingler V., Bartilson,M. and Moore,T. (1993) Cloning and nucleotide sequence of the gene encoding the positive regulator (DmpR) of the phenol catabolic pathway encoded by pVI150 and identification of DmpR as a member of the NtrC family of transcriptional activators. J. Bacteriol., 175, 1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skärfstad E., O’Neill,E., Garmendia,J. and Shingler,V. (2000) Identification of an effector specificity sub-domain within the aromatic responsive regulators DmpR and XylR by DNA shuffling. J. Bacteriol., 182, 3008–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk C.A., Folkers,G.E., Noordman,A.M., Wechselberger,R., van den Brink,N., Boelens,R. and Kaptein,R. (1999) Hinge-helix formation and DNA bending in various lac repressor–operator complexes. EMBO J., 18, 6472–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze C.C., Moore,T. and Shingler,V. (1996) Growth phase-dependent transcription of the σ54-dependent Po promoter controlling the Pseudomonas-derived (methyl)phenol dmp operon of pVI150. J. Bacteriol., 178, 3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorgias C.E. and Wilson, K.S. (1991) A rapid purification procedure of recombinant integration host factor from Escherichia coli.Protein Expr. Purif., 2, 317–320. [DOI] [PubMed] [Google Scholar]
- Wang Y.K., Lee,J.H., Brewer,J.M. and Hoover,T.R. (1997) A conserved region in the σ54-dependent activator DctD is involved in both binding to RNA polymerase and coupling ATP hydrolysis to activation. Mol. Microbiol., 26, 373–386. [DOI] [PubMed] [Google Scholar]
- Watson R.J. (1990) Analysis of the C4-dicarboxylate transport genes of Rhizobium meliloti: nucleotide sequence and deduced products of dctA, dctB, and dctD. Mol. Plant Microbe Interact., 3, 174–181. [DOI] [PubMed] [Google Scholar]
- Weiss D.S., Batut,J., Klose,K.E., Keener,J. and Kustu,S. (1991) The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell, 67, 155–167. [DOI] [PubMed] [Google Scholar]
- Wootton J.C. and Drummond,M.H. (1989) The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng., 2, 535–543. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Heu,S., Yi,J., Lu,Y. and Hutcheson,S.W. (1994) Identification of a putative alternate sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J. Bacteriol., 176, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]