Abstract
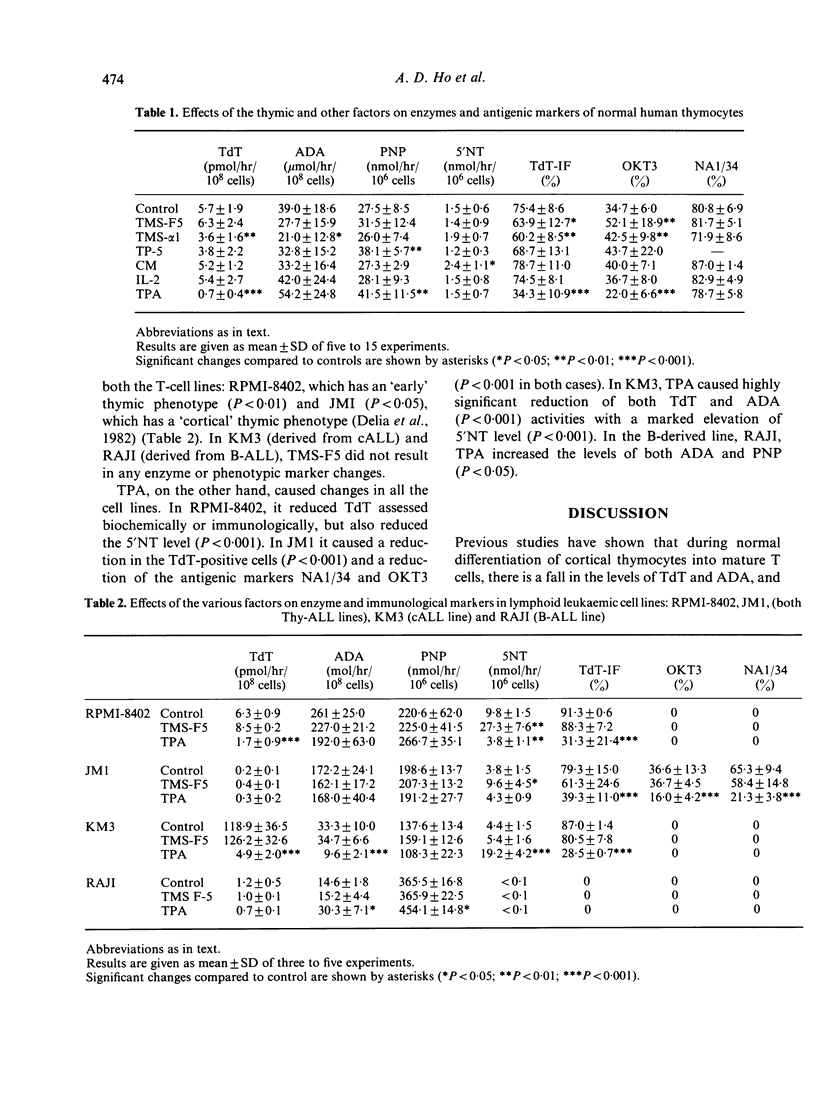
Changes in levels of purine degradative enzymes have been shown to occur during T-cell maturation in both rats and humans with a fall in adenosine deaminase (ADA) and a rise in purine nucleoside phosphorylase (PNP) and 5'-nucleotidase (5'NT) activities. We have investigated the effects of four thymic factors: thymosin fraction 5 (TMS-F5); thymosin alpha 1 (TMS-alpha 1); thymopoietin pentapeptide (TP-5); and thymic conditioned medium (CM) on TdT activity, purine enzyme levels and the phenotypic markers OKT3 (a marker for mature T cells) and NA1/34 (which reacts with immature cortical thymocytes) in human thymocytes and in the lymphoid leukaemic cell lines RPMI-8402 and JM1 (derived from Thy-ALL). All four thymic factors caused one or more maturation change in human thymocytes, e.g. TMS-F5 caused a significant increase in OKT3 expression, TMS-alpha 1 a fall in TdT and ADA activities and a rise in OKT3-positive cells, TP-5 an increase in PNP and CM a rise in 5'NT activity. TMS-F5 also caused a marked elevation of 5'NT in both the T lymphoblastic lines (P less than 0.001). On the other hand the non-physiological phorbol ester, 12-O-tetradecanoyl phorbol acetate (TPA), a tumour promotor with potency of inducing differentiation in some leukaemic cell lines, induced changes in both normal thymocytes and in the leukaemic line JM1 were inconsistent with maturation, e.g. a fall in the percentage of OKT3 cells. These observations suggest that maturation of normal thymocytes might proceed stepwise, each step requiring at least one of the thymic hormones. Although thymosin also induces differentiation changes in a malignant lymphoid line, the pattern of these differs from that induced in their normal counterparts.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A., Wong D. M., Thurman G. B., Low T. L., Goldstein A. L., Sharkis S. J., Goldschneider I. T-lymphocyte maturation: cell surface markers and immune function induced by T-lymphocyte cell-free products and thymosin polypeptides. Ann N Y Acad Sci. 1979;332:81–94. doi: 10.1111/j.1749-6632.1979.tb47100.x. [DOI] [PubMed] [Google Scholar]
- Barton R., Martiniuk F., Hirschhorn R., Goldschneider I. Inverse relationship between adenosine deaminase and purine nucleoside phosphorylase in rat lymphocyte populations. Cell Immunol. 1980 Jan;49(1):208–214. doi: 10.1016/0008-8749(80)90071-4. [DOI] [PubMed] [Google Scholar]
- Cohen A., Dosch H. M., Gelfand E. W. Induction of ecto-5'-nucleotidase activity in human thymocytes. Clin Immunol Immunopathol. 1981 Feb;18(2):287–290. doi: 10.1016/0090-1229(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Cossman J., Neckers L. M., Arnold A., Korsmeyer S. J. Induction of differentiation in a case of common acute lymphoblastic leukemia. N Engl J Med. 1982 Nov 11;307(20):1251–1254. doi: 10.1056/NEJM198211113072006. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Ahmed A., Bollum F. J., Goldstein A. L. Induction of terminal deoxynucleotidyl transferase and Lyt antigens with thymosin: identification of multiple subsets of prothymocytes in mouse bone marrow and spleen. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2469–2473. doi: 10.1073/pnas.78.4.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Low T. L., Thurman G. B., Zatz M. M., Hall N., Chen J., Hu S. K., Naylor P. B., McClure J. E. Current status of thymosin and other hormones of the thymus gland. Recent Prog Horm Res. 1981;37:369–415. doi: 10.1016/b978-0-12-571137-1.50012-8. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Scheid M. P., Boyse E. A., Schlesinger D. H., Van Wauwe J. A synthetic pentapeptide with biological activity characteristic of the thymic hormone thymopoietin. Science. 1979 Jun 22;204(4399):1309–1310. doi: 10.1126/science.451537. [DOI] [PubMed] [Google Scholar]
- Hoffbrand A. V., Ganeshaguru K., Janossy G., Greaves M. F., Catovsky D., Woodruff R. K. Terminal deoxynucleotidyl-transferase levels and membrane phenotypes in diagnosis of acute leukaemia. Lancet. 1977 Sep 10;2(8037):520–523. doi: 10.1016/s0140-6736(77)90662-6. [DOI] [PubMed] [Google Scholar]
- Ma D. D., Sylwestrowicz T. A., Granger S., Massaia M., Franks R., Janossy G., Hoffbrand A. V. Distribution of terminal deoxynucleotidyl transferase and purine degradative and synthetic enzymes in subpopulations of human thymocytes. J Immunol. 1982 Oct;129(4):1430–1435. [PubMed] [Google Scholar]
- Nagasawa K., Mak T. W. Phorbol esters induce differentiation in human malignant T lymphoblasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2964–2968. doi: 10.1073/pnas.77.5.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash L., Good R. A., Hatzfeld A., Goldstein G., Incefy G. S. In vitro differentiation of two surface markers for immature T cells by the synthetic pentapeptide, thymopoietin. J Immunol. 1981 Jan;126(1):150–153. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Gallo R. C. Human T-lymphocyte growth factor: regulation of growth and function of T lymphocytes. Blood. 1981 Mar;57(3):379–394. [PubMed] [Google Scholar]
- Scollay R. G., Butcher E. C., Weissman I. L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980 Mar;10(3):210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Sylwestrowicz T. A., Ma D. D., Murphy P. P., Massaia M., Prentice H. G., Hoffbrand A. V., Greaves M. F. 5'nucleotidase, adenosine deaminase and purine nucleoside phosphorylase activities in acute leukaemia. Leuk Res. 1982;6(4):475–482. doi: 10.1016/0145-2126(82)90004-2. [DOI] [PubMed] [Google Scholar]
- Tidman N., Janossy G., Bodger M., Granger S., Kung P. C., Goldstein G. Delineation of human thymocyte differentiation pathways utilizing double-staining techniques with monoclonal antibodies. Clin Exp Immunol. 1981 Sep;45(3):457–467. [PMC free article] [PubMed] [Google Scholar]
- Wetzel R., Heyneker H. L., Goeddel D. V., Jhurani P., Shapiro J., Crea R., Low T. L., McClure J. E., Thurman G. B., Goldstein A. L. Production of biologically active N alpha-desacetylthymosin alpha 1 in Escherichia coli through expression of a chemically synthesized gene. Biochemistry. 1980 Dec 23;19(26):6096–6104. doi: 10.1021/bi00567a023. [DOI] [PubMed] [Google Scholar]


