Abstract
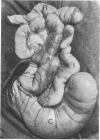
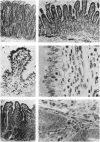
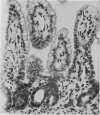
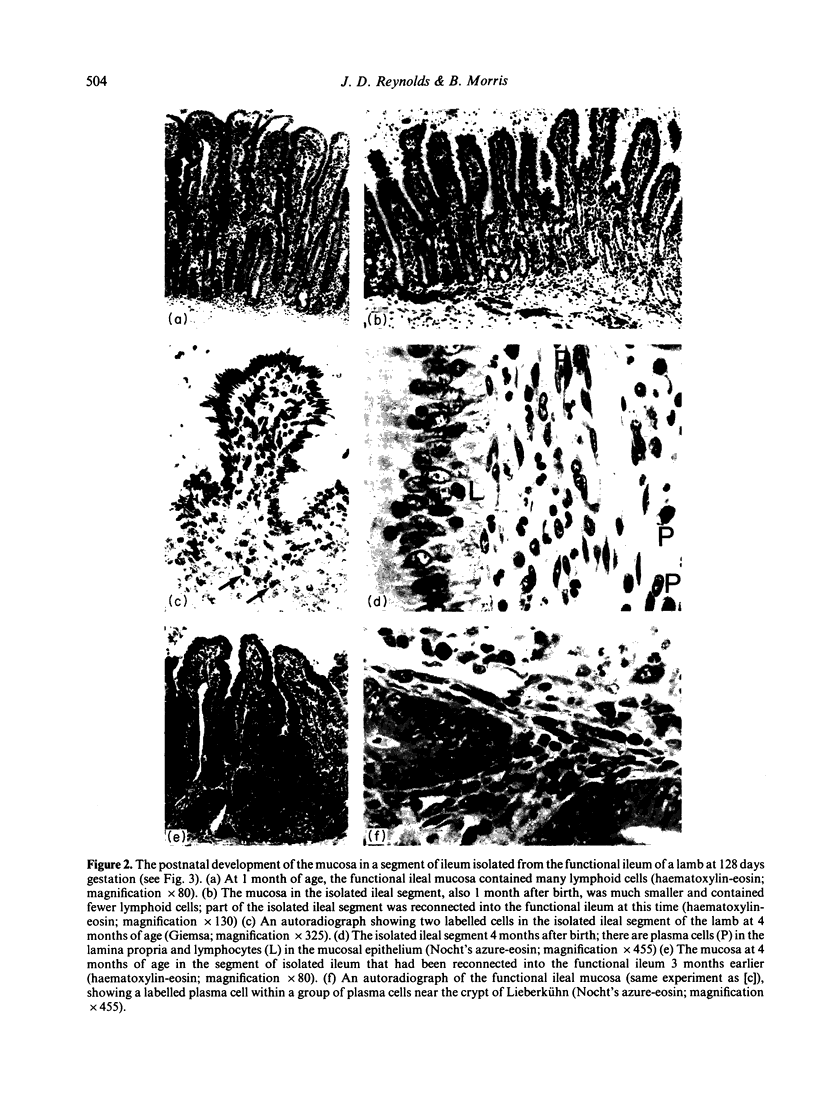
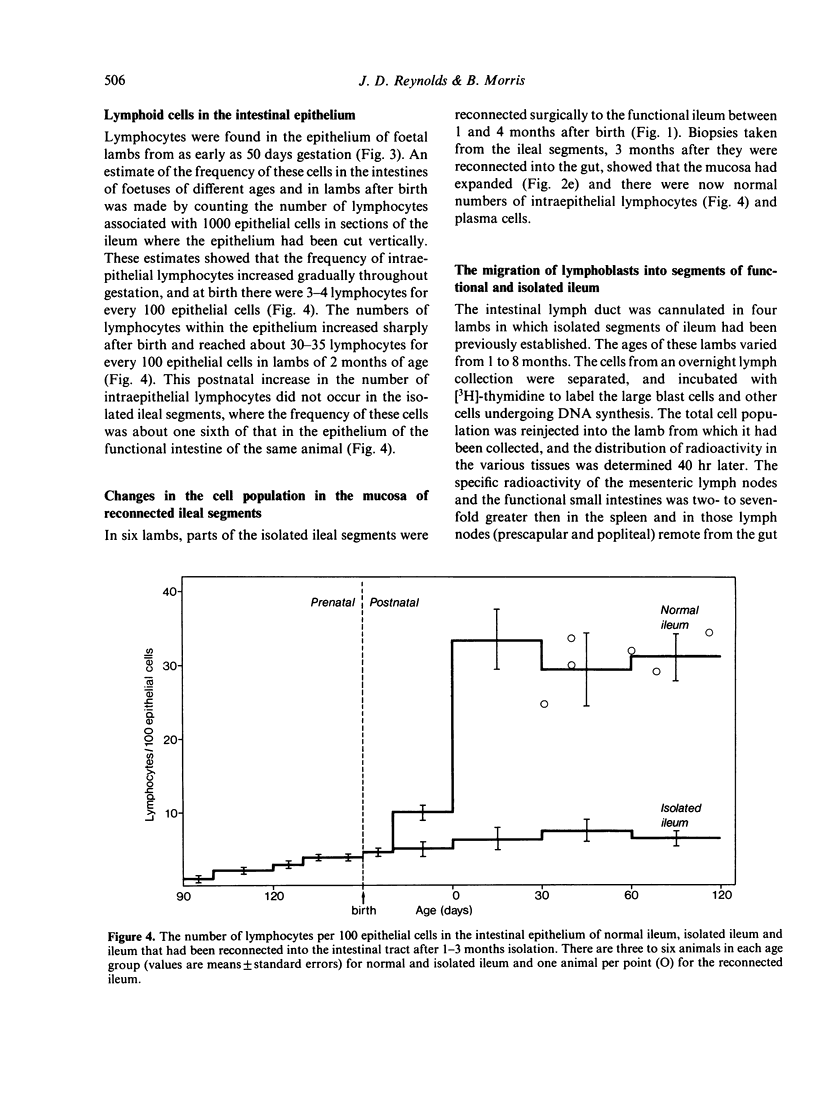
The number and type of lymphoid cells in the intestinal mucosa of lambs change during the first weeks after birth. The influence of gut function on these changes was examined by comparing the evolution of lymphoid cell populations in normal ileum with that in lengths of ileum which had been isolated surgically from the functional intestinal tract of the lamb before birth. The isolated lengths of ileum had a normal blood and nerve supply and they remained healthy throughout a period of at least 2 years, although they did not have a normal histological development. In comparison with normal ileum, the villi of the isolated ileal segments were much smaller and there were many fewer intraepithelial lymphocytes; the lamina propria had significantly fewer lymphocytes than the functional ileum and only a few plasma cells. When isolated ileal segments were reconnected into the intestinal tract after having been isolated from it for 1-3 months, the histology of the mucosa reverted to that of the normal gut, with the same number and types of lymphoid cells. Radiolabelled lymphoblasts collected from intestinal lymph and injected intravenously accumulated to only a small extent in isolated segments of ileum compared with either the normal or the reconnected segments of ileum. This suggested that the paucity of lymphocytes in the mucosa of the isolated segments was due to a reduced extravasation of these cells there. The influence which the gut contents exert on the lymphoid cell population in the mucosa is probably associated with antigenic stimulation but may also be related to other factors concerned in the normal digestive functions of the gut.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fahey K. J., Morris B. Humoral immune responses in foetal sheep. Immunology. 1978 Oct;35(4):651–661. [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes in the small intestine of the mouse. Clin Exp Immunol. 1972 Dec;12(4):477–488. [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Hopkins J., Reynolds J. Studies of efferent lymph cells from nodes stimulated with oxazolone. Immunology. 1980 Feb;39(2):141–149. [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Parry D. M., Smith M. E. The distribution and differentiation of lymph-borne immunoblasts after intravenous injection into syngeneic recipients. Cell Tissue Kinet. 1972 May;5(3):269–281. doi: 10.1111/j.1365-2184.1972.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Hay J. B., Hobbs B. B. The flow of blood to lymph nodes and its relation to lymphocyte traffic and the immune response. J Exp Med. 1977 Jan 1;145(1):31–44. doi: 10.1084/jem.145.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES A. K., MORRIS B. Surgical techniques for the collection of lymph from unanaesthetized sheep. Q J Exp Physiol Cogn Med Sci. 1961 Jul;46:199–205. doi: 10.1113/expphysiol.1961.sp001536. [DOI] [PubMed] [Google Scholar]
- Moore A. R., Hall J. G. Evidence for a primary association between immunoblasts and small gut. Nature. 1972 Sep 15;239(5368):161–162. doi: 10.1038/239161a0. [DOI] [PubMed] [Google Scholar]
- Ottaway C. A., Parrott D. M. Regional blood flow and the localization of lymphoblasts in the small intestine of the mouse. I. Examination of normal small intestine. Immunology. 1980 Dec;41(4):955–961. [PMC free article] [PubMed] [Google Scholar]
- Parrott D. M., Ferguson A. Selective migration of lymphocytes within the mouse small intestine. Immunology. 1974 Mar;26(3):571–588. [PMC free article] [PubMed] [Google Scholar]
- Rose M. L., Parrott D. M., Bruce R. G. Migration of lymphoblasts to the small intestine. II. Divergent migration of mesenteric and peripheral immunoblasts to sites of inflammation in the mouse. Cell Immunol. 1976 Nov;27(1):36–46. doi: 10.1016/0008-8749(76)90151-9. [DOI] [PubMed] [Google Scholar]
- Röpke C., Everett N. B. Kinetics of intraepithelial lymphocytes in the small intestine of thymus-deprived mice and antigen-deprived mice. Anat Rec. 1976 May;185(1):101–108. doi: 10.1002/ar.1091850110. [DOI] [PubMed] [Google Scholar]
- Smeaton T. C., Cole G. J., Simpson-Morgan M. W., Morris B. Techniques for the long-term collection of lymph from the unanaesthetized foetal lamb in utero. Aust J Exp Biol Med Sci. 1969 Oct;47(5):565–572. doi: 10.1038/icb.1969.150. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Isselbacher K. J. Protein uptake by the intestine: evidence for absorption of intact macromolecules. Gastroenterology. 1974 May;66(5):987–992. [PubMed] [Google Scholar]