Abstract
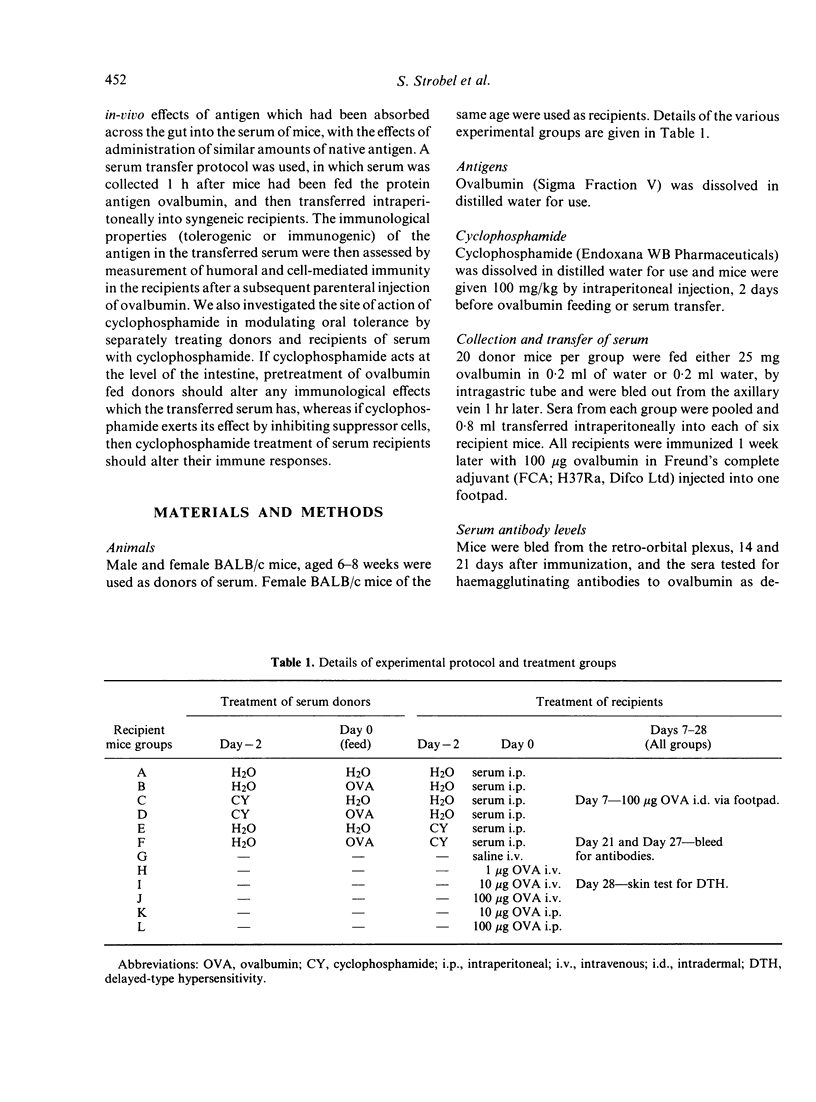
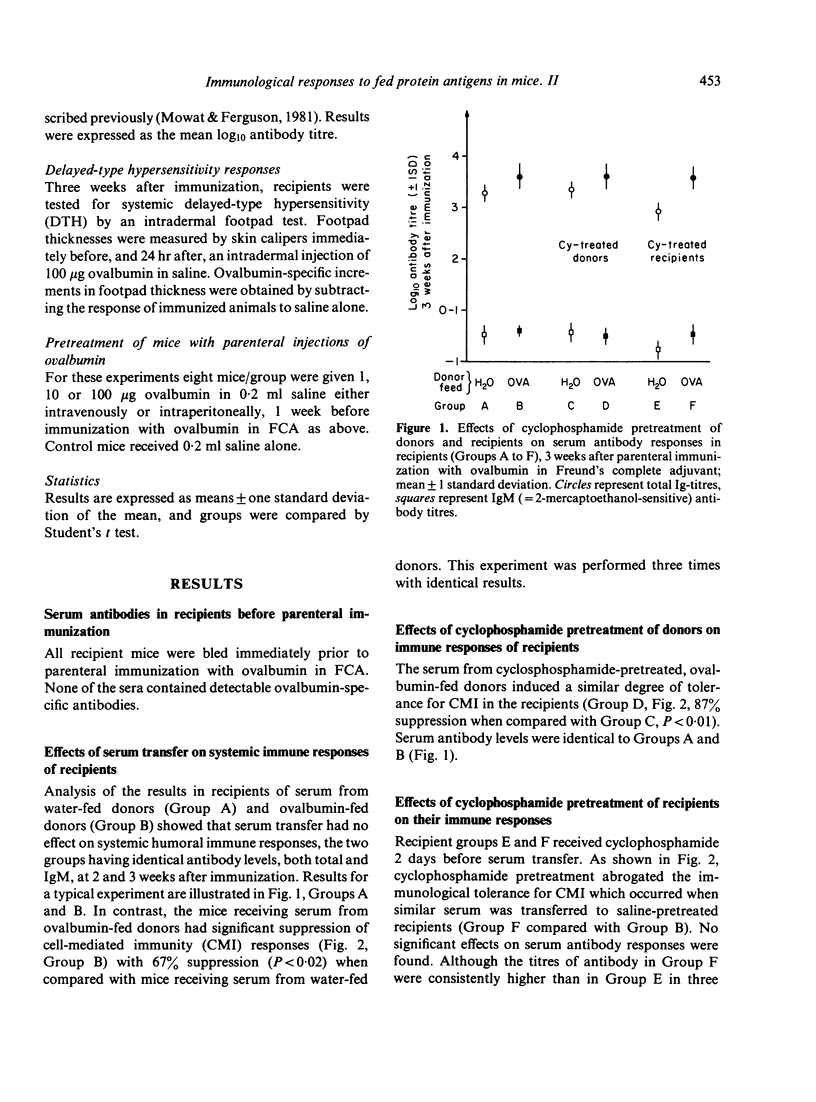
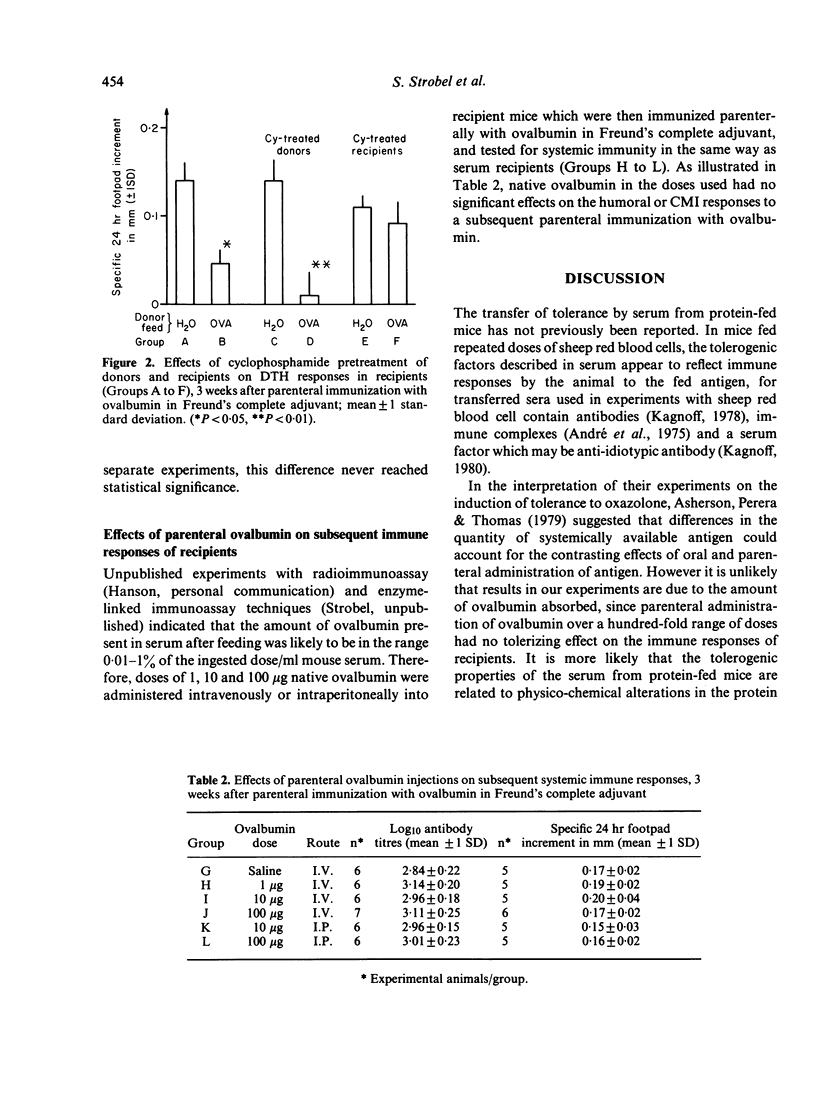
Mice fed ovalbumin develop specific systemic hyporesponsiveness. This oral tolerance is abrogated by cyclophosphamide pretreatment, and the mechanism of abrogation could be either via T suppressor cells or via damage to the gut epithelium. A serum transfer protocol was used to examine the site of action of cyclophosphamide in this system. Serum was collected from ovalbumin-fed mice and transferred into recipients which were then parenterally immunized with ovalbumin in Freund's complete adjuvant. Serum transfer suppressed the delayed-type hypersensitivity (DTH) responses but not the antibody responses of the recipients. Cyclophosphamide pretreatment (100 mg/kg) of recipients (but not of donors) abrogated this suppressor effect. Parenteral administration of ovalbumin in a range of doses did not induce immunological hyporesponsiveness. It is suggested that absorption across the gut mucosa leads to generation of fragments of ovalbumin that induce suppressor cells selective for DTH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., Heremans J. F., Vaerman J. P., Cambiaso C. L. A mechanism for the induction of immunological tolerance by antigen feeding: antigen-antibody complexes. J Exp Med. 1975 Dec 1;142(6):1509–1519. doi: 10.1084/jem.142.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Perera M. A., Thomas W. R. Contact sensitivity and the DNA response in mice to high and low doses of oxazolone: low dose unresponsiveness following painting and feeding and its prevention by pretreatment with cyclophosphamide. Immunology. 1979 Mar;36(3):449–459. [PMC free article] [PubMed] [Google Scholar]
- Dosa S., Pesce A. J., Ford D. J., Muckerheide A., Michael J. G. Immunological properties of peptic fragments of bovine serum albumin. Immunology. 1979 Nov;38(3):509–517. [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F. Effects of antigen-feeding on intestinal and systemic immune responses. III. Antigen-specific serum-mediated suppression of humoral antibody responses after antigen feeding. Cell Immunol. 1978 Sep 15;40(1):186–203. doi: 10.1016/0008-8749(78)90326-x. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. II. Antigen-specific helper and suppressor factors produced by spleen cells of rats fed sheep erythrocytes. J Immunol. 1980 Sep;125(3):1044–1047. [PubMed] [Google Scholar]
- Miller S. D., Hanson D. G. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979 Nov;123(5):2344–2350. [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Hypersensitivity in the small intestinal mucosa. V. Induction of cell-mediated immunity to a dietary antigen. Clin Exp Immunol. 1981 Mar;43(3):574–582. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Ngan J., Kind L. S. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978 Mar;120(3):861–865. [PubMed] [Google Scholar]
- Parks D. E., Weigle W. O. Maintenance of immunologic unresponsiveness to human gamma-globulin: evidence for irreversible inactivation in B lymphocytes. J Immunol. 1980 Mar;124(3):1230–1236. [PubMed] [Google Scholar]
- Richman L. K., Chiller J. M., Brown W. R., Hanson D. G., Vaz N. M. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphoyctes by intragastric administration of soluble proteins. J Immunol. 1978 Dec;121(6):2429–2434. [PubMed] [Google Scholar]
- Röllinghoff M., Starzinski-Powitz A., Pfizenmaier K., Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977 Feb 1;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Askenase P. W., Gershon R. K. Regulation of delayed-type hypersensitivity reactions by cyclophosphamide-sensitive T cells. J Immunol. 1978 Oct;121(4):1573–1577. [PubMed] [Google Scholar]
- Vives J., Parks D. E., Weigle W. O. Immunologic unresponsiveness after gastric administration of human gamma-globulin: antigen requirements and cellular parameters. J Immunol. 1980 Oct;125(4):1811–1816. [PubMed] [Google Scholar]


