Abstract
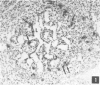
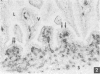
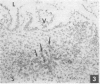
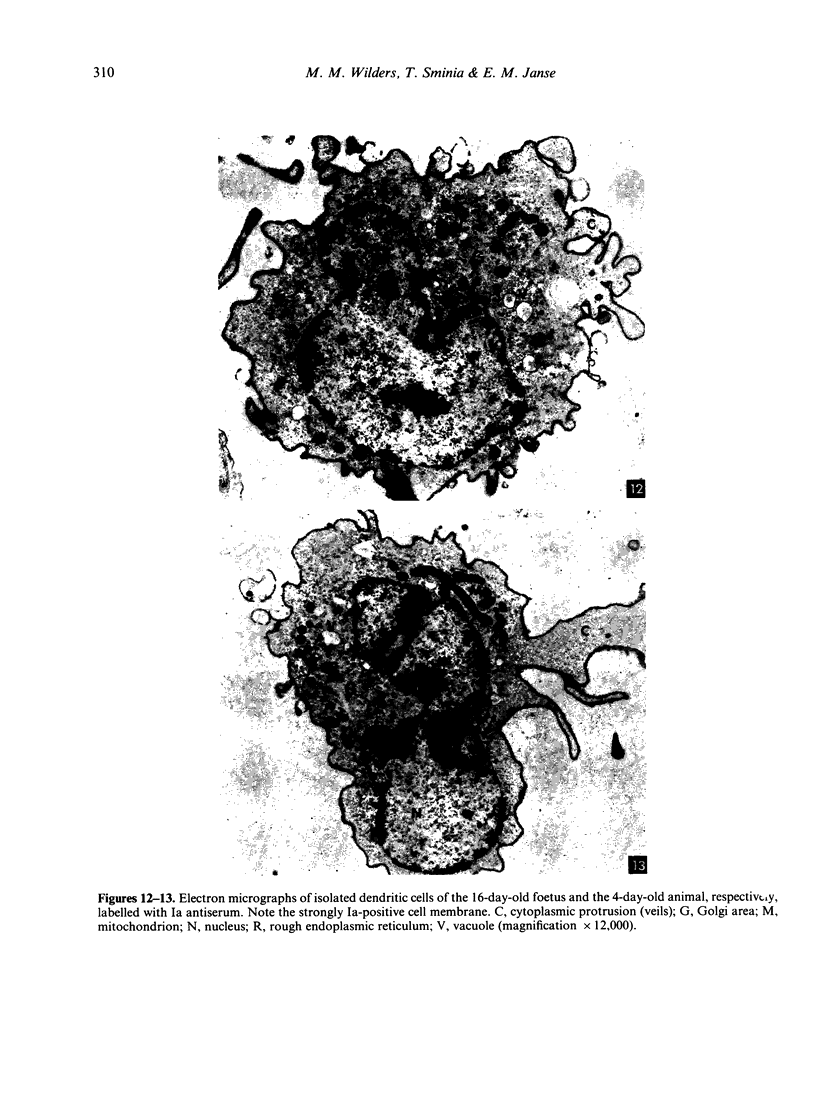
The ontogeny of rat Peyer's patches was studied with special reference to Ia-positive dendritic cells. The 16-day-old foetus contained large Ia-positive dendritic cells randomly distributed throughout the gut wall and mesentery. In cell suspensions prepared from the foetal gut, these cells showed the typical movement pattern of veiled cells, and they shared ultrastructural features with these and other antigen-presenting cells. The gut was found to be the first organ in which large numbers of these Ia-positive dendritic cells were found. At 16 days gestation, the Ia-positive dendritic cells were randomly distributed throughout the primitive gut wall, but at later stages accumulated in structures which were clearly recognizable as Peyer's patches from 20 days gestation onwards. T and B lymphocytes could be seen on the day of birth at the earliest, at first randomly distributed throughout the Peyer's patch, but afterwards concentrating in separate T- and B-cell regions. Two weeks after birth Peyer's patches consisted of densely populated B-cell nodules, mainly comprising surface IgM (sIgM) positive cells, and internodular T-cell regions. Ia-positive dendritic cells were situated between the epithelial cells of the dome area, just underneath this epithelium in a loose reticular area and in the internodular T-cell regions. These Ia-positive dendritic cells were acid phosphatase negative or only weakly positive. Some Ia-positive dendritic cells were also present in small intestinal villi at all ages studied. However, these cells started to express an increasing acid phosphatase activity in the 2 weeks following birth. Thus they resemble macrophages, while Peyer's patch dendritic cells show characteristics of antigen-presenting cells.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Bland P. W., Richens E. R., Britton D. C., Lloyd J. V. Isolation and purification of human large bowel mucosal lymphoid cells: effect of separation technique on functional characteristics. Gut. 1979 Dec;20(12):1037–1046. doi: 10.1136/gut.20.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D. Characterization of nonlymphoid cells in rat spleen, with special reference to strongly Ia-positive branched cells in T-cell areas. J Reticuloendothel Soc. 1982 Sep;32(3):167–178. [PubMed] [Google Scholar]
- Drexhage H. A., Mullink H., de Groot J., Clarke J., Balfour B. M. A study of cells present in peripheral lymph of pigs with special reference to a type of cell resembling the Langerhans cell. Cell Tissue Res. 1979 Nov;202(3):407–430. doi: 10.1007/BF00220434. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Köhler Y. G., Hoefsmit E. C. Interdigitating cells and macrophages in the acute involuting rat thymus. An electron-microscopic study on phagocytic activity and population development. Cell Tissue Res. 1982;224(2):291–301. doi: 10.1007/BF00216874. [DOI] [PubMed] [Google Scholar]
- Kaiserling E., Lennert K. Die interdigitierende Reticulumzelle im menschlichen Lymphknoten. Eine spezifische Zelle der thymusabhängigen Region. Virchows Arch B Cell Pathol. 1974;16(1):51–61. doi: 10.1007/BF02894063. [DOI] [PubMed] [Google Scholar]
- Katz S. I., Tamaki K., Sachs D. H. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979 Nov 15;282(5736):324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- Pugh C. W., MacPherson G. G. Non-lymphoid cells from rat intestinal lymph. Adv Exp Med Biol. 1982;149:781–789. doi: 10.1007/978-1-4684-9066-4_108. [DOI] [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Thorbecke G. J., Baer R. L., Rosenthal S. A., Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976 Aug;25(2):137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- Spry C. J., Pflug A. J., Janossy G., Humphrey J. H. Large mononuclear (veiled) cells like 'Ia-like' membrane antigens in human afferent lympn. Clin Exp Immunol. 1980 Mar;39(3):750–755. [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl G., Katz S. I., Clement L., Green I., Shevach E. M. Immunologic functions of Ia-bearing epidermal Langerhans cells. J Immunol. 1978 Nov;121(5):2005–2013. [PubMed] [Google Scholar]
- Thorbecke G. J., Silberberg-Sinakin I., Flotte T. J. Langerhans cells as macrophages in skin and lymphoid organs. J Invest Dermatol. 1980 Jul;75(1):32–43. doi: 10.1111/1523-1747.ep12521083. [DOI] [PubMed] [Google Scholar]
- Veerman A. J. On the interdigitating cells in the thymus-dependent area of the rat spleen: a relation between the mononuclear phagocyte system and T-lymphocytes. Cell Tissue Res. 1974 Apr 11;148(2):247–257. doi: 10.1007/BF00224586. [DOI] [PubMed] [Google Scholar]
- Wilders M. M., Drexhage H. A., Weltevreden E. F., Mullink H., Duijvestijn A., Meuwissen S. G. Large mononuclear Ia-positive veiled cells in Peyer's patches. I. Isolation and characterization in rat, guinea-pig and pig. Immunology. 1983 Mar;48(3):453–460. [PMC free article] [PubMed] [Google Scholar]
- Wilders M. M., Sminia T., Plesch B. E., Drexhage H. A., Weltevreden E. F., Meuwissen S. G. Large mononuclear Ia-positive veiled cells in Peyer's patches. II. Localization in rat Peyer's patches. Immunology. 1983 Mar;48(3):461–467. [PMC free article] [PubMed] [Google Scholar]
- Wolff K. The langerhans cell. Curr Probl Dermatol. 1972;4:79–145. [PubMed] [Google Scholar]