Abstract
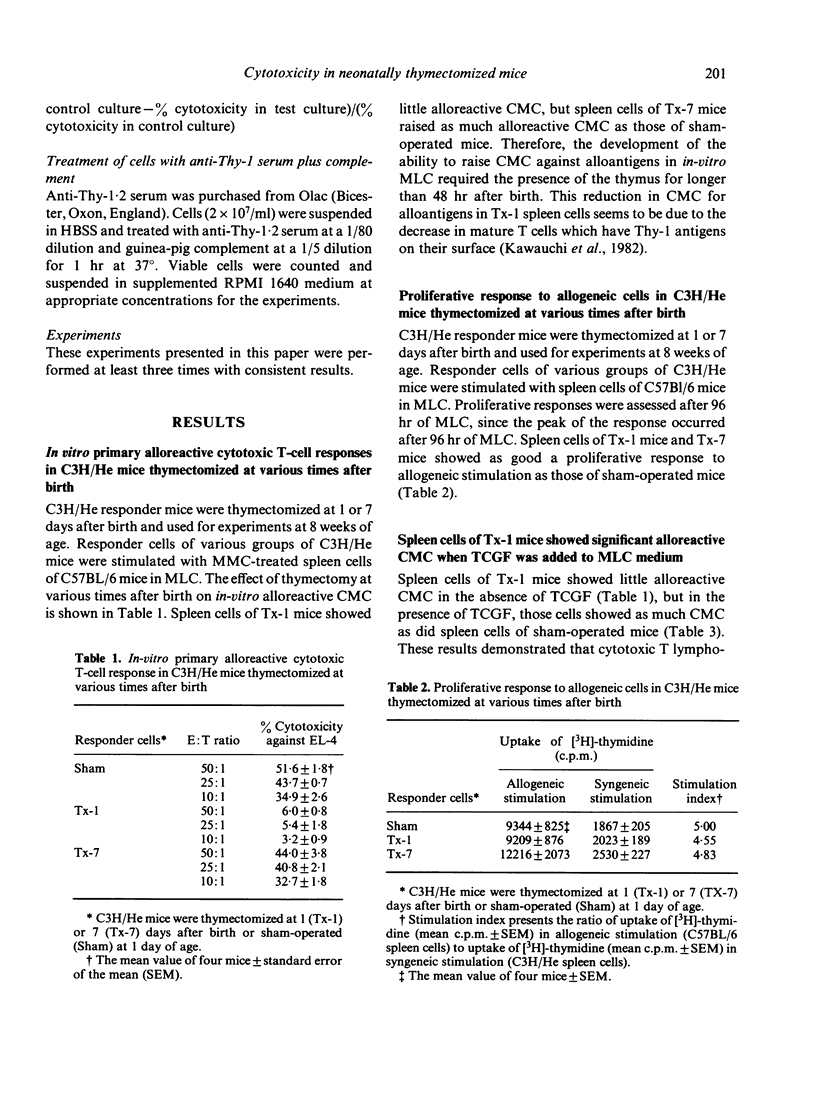
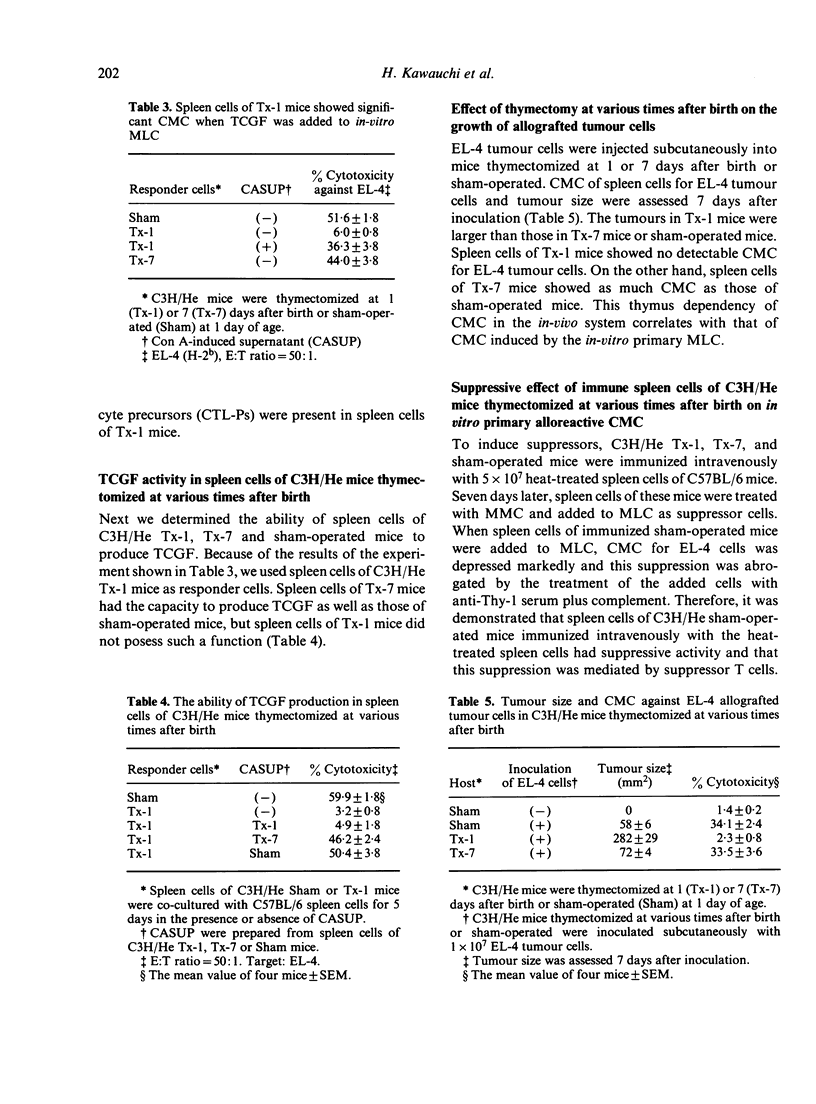
The effect of neonatal thymectomy at various times after birth (Tx-1, Tx-7) on effector and suppressor T cells responsible for cell-mediated cytotoxicity (CMC) for allogenic antigens was determined. Following in-vitro primary mixed lymphocyte cultures, in the absence of T-cell growth factor (TCGF), alloreactive CMC was not detected in spleen cells of Tx-1 mice, but was detected in spleen cells of Tx-7 mice at as high levels as in those of sham-operated mice. However, in the presence of TCGF, as much alloreactive CMC was detected in spleen cells of Tx-1 mice as in those of Tx-7 mice. Furthermore, TCGF production was not detected in spleen cells of Tx-1 mice but was detected in those of Tx-7 mice. In in-vivo experiments, inhibition of allogeneic tumour growth and CMC in spleen cells showed the same pattern as in in-vitro experiments. These results support the concept that the reduction of CMC in Tx-1 mice might be due to a defect in helper function (TCGF-producing capacity) rather than to a defect in cytotoxic T lymphocytes and/or cytotoxic T lymphocyte precursors. Alloreactive suppressor T cells could not be induced in spleen cells of Tx-1 mice but were induced in spleen cells of Tx-7 mice. Therefore, it was suggested that alloreactive suppressor T cells require the presence of the thymus for 7 days after birth in their development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyris B. F. Nature of neonatal splenic suppressor cells in the mouse. Cell Immunol. 1982 Jan 15;66(2):352–359. doi: 10.1016/0008-8749(82)90185-x. [DOI] [PubMed] [Google Scholar]
- Brondz B. D., Karaulov A. V., Abronina I. F., Blandova Z. K. Requirements for induction of specific suppressor T cells and detection of their H-2 antigen-binding receptors by fractionation on target cell monolayers. Scand J Immunol. 1981;13(6):517–534. doi: 10.1111/j.1365-3083.1981.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Simpson E., Sato V. L., Fathman C. G., Herzenberg L. A. Characterization of subpopulations of T lymphocytes. I. Separation and functional studies of peripheral T-cells binding different amounts of fluorescent anti-Thy 1.2 (theta) antibody using a fluorescence-activated cell sorter (FACS). Cell Immunol. 1975 Jan;15(1):180–196. doi: 10.1016/0008-8749(75)90174-4. [DOI] [PubMed] [Google Scholar]
- Chiu K. M., Faanes R. B., Choi Y. S. Activation of specific suppressor cells with heat-treated allogeneic tumor cells. Cell Immunol. 1980 Feb;49(2):283–292. doi: 10.1016/0008-8749(80)90030-1. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., DALMASSO A. P., MARTINEZ C., ARCHER O. K., PIERCE J. C., PAPERMASTER B. W. The role of the thymus in development of immunologic capacity in rabbits and mice. J Exp Med. 1962 Nov 1;116:773–796. doi: 10.1084/jem.116.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hünig T., Bevan M. J. Specificity of cytotoxic T cells from athymic mice. J Exp Med. 1980 Sep 1;152(3):688–702. doi: 10.1084/jem.152.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi H., Shimamoto Y., Taniguchi K., Kubo C., Nomoto K. Differences in thymus dependency among the alloreactive T-cell subpopulations in their development. Cell Immunol. 1982 Jun;70(1):76–84. doi: 10.1016/0008-8749(82)90134-4. [DOI] [PubMed] [Google Scholar]
- Komuro K., Boyse E. A. Induction of T lymphocytes from precursor cells in vitro by a product of the thymus. J Exp Med. 1973 Aug 1;138(2):479–482. doi: 10.1084/jem.138.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. F. Immunological function of the thymus. Lancet. 1961 Sep 30;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Johnson B. M. Ontogeny of mouse lymphocyte function. II. Development of the ability to produce antibody is modulated by T lymphocytes. J Exp Med. 1975 Jan 1;141(1):216–226. doi: 10.1084/jem.141.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Theta-bearing lymphocytes in nude mice. Nature. 1973 Dec 7;246(5432):350–351. doi: 10.1038/246350a0. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Goldstein G., Boyse E. A., Schield M. P. T cell development in normal and thymopentin-treated nude mice. J Exp Med. 1982 Oct 1;156(4):1057–1064. doi: 10.1084/jem.156.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Alter B. J., Bach F. H. Lyt phenotypes of alloreactive precursor and effector cytotoxic T lymphocytes. J Immunol. 1981 Jan;126(1):353–358. [PubMed] [Google Scholar]
- SJODIN K., DALMASSO A. P., SMITH J. M., MARTINEZ C. THYMECTOMY IN NEWBORN AND ADULT MICE. Transplantation. 1963 Oct;1:521–525. doi: 10.1097/00007890-196301040-00011. [DOI] [PubMed] [Google Scholar]
- Sato V. L., Waksal S. D., Herzenberg L. A. Identification and separation of pre T-cells from nu/nu mice: differentiation by preculture with thymic reticuloepithelial cells. Cell Immunol. 1976 Jun 1;24(1):173–185. doi: 10.1016/0008-8749(76)90142-8. [DOI] [PubMed] [Google Scholar]
- Shimamoto Y., Taniguchi K., Kubo C., Nomoto K. Differences in thymus-dependency among various T-cell functions. Immunology. 1980 Sep;41(1):167–178. [PMC free article] [PubMed] [Google Scholar]
- Singh U., Owen J. J. Studies on the effect of various agents on the maturation of thymus stem cells. Eur J Immunol. 1975 Apr;5(4):286–288. doi: 10.1002/eji.1830050414. [DOI] [PubMed] [Google Scholar]


