Abstract
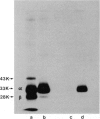
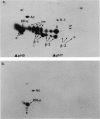
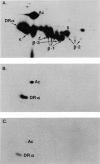
Two monoclonal antibodies, TAL-1B5 and TAL-3C3, specific for human Ia alpha-chain subunits have been produced by fusing P3/NSI/1-Ag4-1 mouse myeloma cells with spleen cells from a BALB/c mouse immunized with purified alpha-chains. Specificity for the alpha-chain subunits was initially established using a solid-phase radioimmunoassay. Indirect binding assays demonstrated that TAL-1B5 bound strongly to all human B lymphoblastoid lines tested and to CLLs, but only weakly to PBL-B cells and not to PBL-T cells or the T-cell lines Molt 4 and HSB-2. TAL-3C3 bound only weakly to B lymphoblastoid lines and not to CLLs or PBL-B cells. From 125I cell surface-labelled lysates TAL-1B5 immunoprecipitated a 33,000(alpha):28,000(beta) Ia dimer, but TAL-3C3 failed to immunoprecipitate cell surface molecules. Under denaturing conditions, however, both TAL-1B5 and TAL-3C3 immunoprecipitated the 33,000 alpha-chain subunit. Competitive inhibition studies demonstrated that both monoclonal antibodies recognize the same or spatially related alpha-chain antigenic determinants with some slight cross-reactivity against beta-chains. 2D-NEPHGE/SDS-PAGE analysis of TAL-1B5 immunoprecipitates from [35S]-methionine biosynthetically labelled cells revealed the presence of a number of alpha-chain spots in association with beta-chain products of three previously described loci (beta-1, beta-2, beta-3) suggesting that this antibody recognizes an antigenic site common to those human Ia alpha-chains so far identified.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. A., Flowers A., Davis B. J. Direct implantation and serial transplantation of human acute lymphoblastic leukemia in hamsters, SB-2. Cancer Res. 1968 Jun;28(6):1121–1125. [PubMed] [Google Scholar]
- Allison J. P., Walker L. E., Russell W. A., Pellegrino M. A., Ferrone S., Reisfeld R. A., Frelinger J. A., Silver J. Murine Ia and human DR antigens: homology of amino-terminal sequences. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3953–3956. doi: 10.1073/pnas.75.8.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer J. G. Ia antigens: definition of the HLA-DRw specificities. Br Med Bull. 1978 Sep;34(3):233–240. doi: 10.1093/oxfordjournals.bmb.a071503. [DOI] [PubMed] [Google Scholar]
- Bono M. R., Strominger J. L. Direct evidence of homology between human DC-1 antigen and murine I-A molecules. Nature. 1982 Oct 28;299(5886):836–840. doi: 10.1038/299836a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- De Kretser T. A., Crumpton M. J., Bodmer J. G., Bodmer W. F. Two-dimensional gel analysis of the polypeptides precipitated by a polymorphic HLA-DR1,2,w6 monoclonal antibody: evidence for a third locus. Eur J Immunol. 1982 Jul;12(7):600–606. doi: 10.1002/eji.1830120713. [DOI] [PubMed] [Google Scholar]
- Duquesnoy R. J., Marrari M., Annen K. Identification of an HLA-DR-associated system of B-cell alloantigens. Transplant Proc. 1979 Dec;11(4):1757–1760. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B. B., Deane D. L., Steel C. M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982 Nov;12(11):942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. P., Meo T., Riethmüller G., Schendel D. J., Wank R. Direct demonstration of an HLA-DR allotypic determinant on the low molecular weight (beta) subunit using a mouse monoclonal antibody specific for DR3. J Exp Med. 1982 Jul 1;156(1):104–111. doi: 10.1084/jem.156.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Travers P. J., Carey J., Grosveld F., Jenkins J., Bodmer W. F. Sequence of an HLA-DR alpha-chain cDNA clone and intron-exon organization of the corresponding gene. Nature. 1982 Oct 21;299(5885):750–752. doi: 10.1038/299750a0. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Cresswell P. Human B cell alloantigens; alpha subunit variability. J Immunol. 1982 May;128(5):1999–2003. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Palacios R., Claesson L., Möller G., Peterson P. A., Möller E. The alpha chain, not the beta chain of HLA-DR antigens participates in activation of T cells in autologous mixed lymphocyte reaction. Immunogenetics. 1982;15(4):341–356. doi: 10.1007/BF00364258. [DOI] [PubMed] [Google Scholar]
- Palacios R. Role of individual chains of HLA-DR antigens in activation of T cells induced by alloantigens. Immunogenetics. 1981;14(3-4):309–322. doi: 10.1007/BF00342200. [DOI] [PubMed] [Google Scholar]
- Quaranta V., Tanigaki N., Ferrone S. Distribution of antigenic determinants recognized by three monoclonal antibodies (Q2/70, Q5/6 and Q5/13) on human Ia-like alloantigens and on their subunits. Immunogenetics. 1981;12(1-2):175–182. doi: 10.1007/BF01561660. [DOI] [PubMed] [Google Scholar]
- Ragland W. L., Pace J. L., Kemper D. L. Fluorometric scanning of fluorescamine-labeled proteins in polyacrylamide gels. Anal Biochem. 1974 May;59(1):24–33. doi: 10.1016/0003-2697(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Lampson L. A., Strominger J. L. Analysis of HLA-DR antigens by using monoclonal antibodies: recognition of conformational differences in biosynthetic intermediates. J Immunol. 1981 Oct;127(4):1403–1410. [PubMed] [Google Scholar]
- Shackelford D. A., Mann D. L., van Rood J. J., Ferrara G. B., Strominger J. L. Human B-cell alloantigens DC1, MT1, and LB12 are identical to each other but distinct from the HLA-DR antigen. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4566–4570. doi: 10.1073/pnas.78.7.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., DeMars R., Schlossman S. F., Smith P. L., Lampson L. A., Nadler L. M. Serologic identification of the human secondary B cell antigens. Correlations between function, genetics, and structure. J Exp Med. 1982 Sep 1;156(3):731–743. doi: 10.1084/jem.156.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Kavathas P., Pollack M. S., Charmot D., Mawas C. Family studies define a new histocompatibility locus, SB, between HLA-DR and GLO. Nature. 1981 Oct 29;293(5835):745–747. doi: 10.1038/293745a0. [DOI] [PubMed] [Google Scholar]
- Snary D., Barnstable C. J., Bodmer W. F., Crumpton M. J. Molecular structure of human histocompatibility antigens: the HLA-C series. Eur J Immunol. 1977 Aug;7(8):580–585. doi: 10.1002/eji.1830070816. [DOI] [PubMed] [Google Scholar]
- Stocker J. W., Heusser C. H. Methods for binding cells to plastic: application to a solid-phase radioimmunoassay for cell-surface antigens. J Immunol Methods. 1979;26(1):87–95. doi: 10.1016/0022-1759(79)90044-9. [DOI] [PubMed] [Google Scholar]
- Stähli C., Staehelin T., Miggiano V., Schmidt J., Häring P. High frequencies of antigen-specific hybridomas: dependence on immunization parameters and prediction by spleen cell analysis. J Immunol Methods. 1980;32(3):297–304. doi: 10.1016/0022-1759(80)90194-5. [DOI] [PubMed] [Google Scholar]
- Termijtelen A., Boettcher B., Bradley B. A., D'Amaro J., van Leeuwen A., van Rood J. J. DR typing in Australian Aborigines. An indication for a second locus in the HLA--D region defined by serology. Tissue Antigens. 1980 Aug;16(2):140–146. doi: 10.1111/j.1399-0039.1980.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Tosi R., Tanigaki N., Centis D., Ferrara G. B., Pressman D. Immunological dissection of human Ia molecules. J Exp Med. 1978 Dec 1;148(6):1592–1611. doi: 10.1084/jem.148.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead A. S., Sim R. B., Bodmer W. F. A monoclonal antibody against human complement component C3: the production of C3 by human cells in vitro. Eur J Immunol. 1981 Feb;11(2):140–146. doi: 10.1002/eji.1830110215. [DOI] [PubMed] [Google Scholar]
- de Kretser T. A., Bodmer J. G., Bodmer W. F. The separation of cell populations using monoclonal antibodies attached to sepharose. Tissue Antigens. 1980 Oct;16(4):317–325. doi: 10.1111/j.1399-0039.1980.tb00313.x. [DOI] [PubMed] [Google Scholar]
- de Kretser T. A., Crumpton M. J., Bodmer J. G., Bodmer W. F. Demonstration of two distinct light chains in HLA-DR-associated antigens by two-dimensional gel electrophoresis. Eur J Immunol. 1982 Mar;12(3):214–221. doi: 10.1002/eji.1830120309. [DOI] [PubMed] [Google Scholar]