Abstract
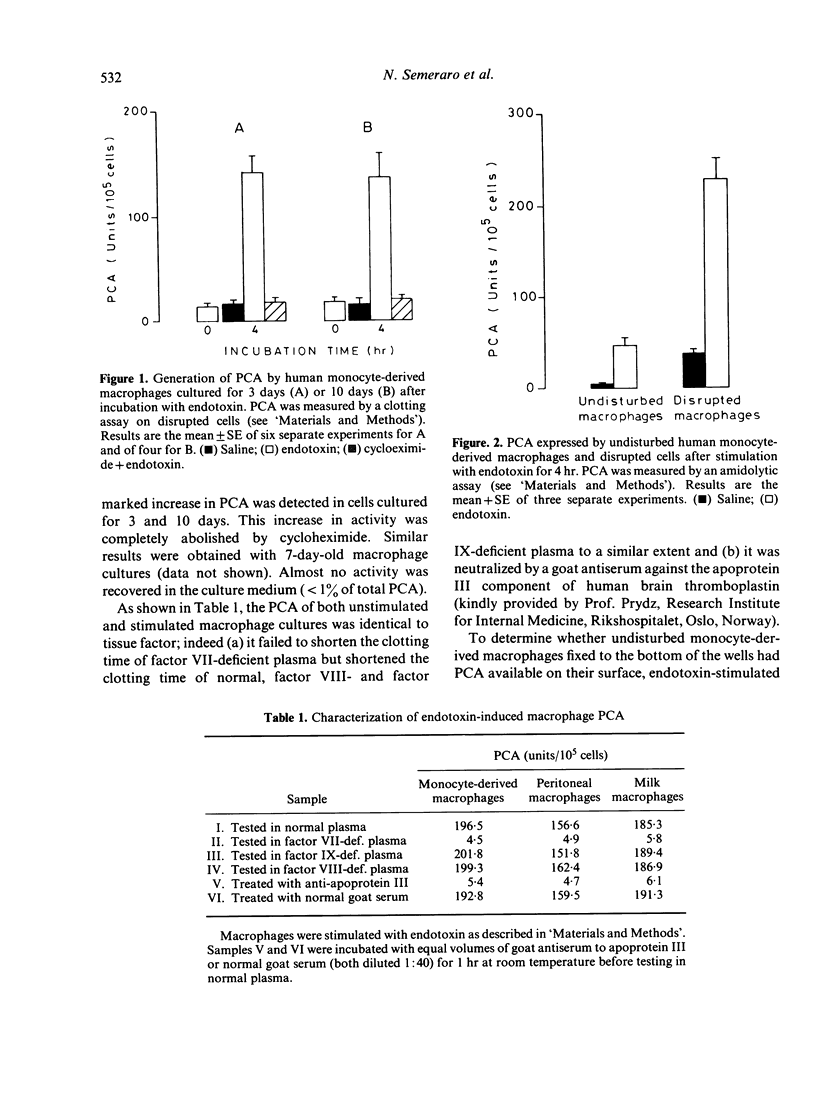
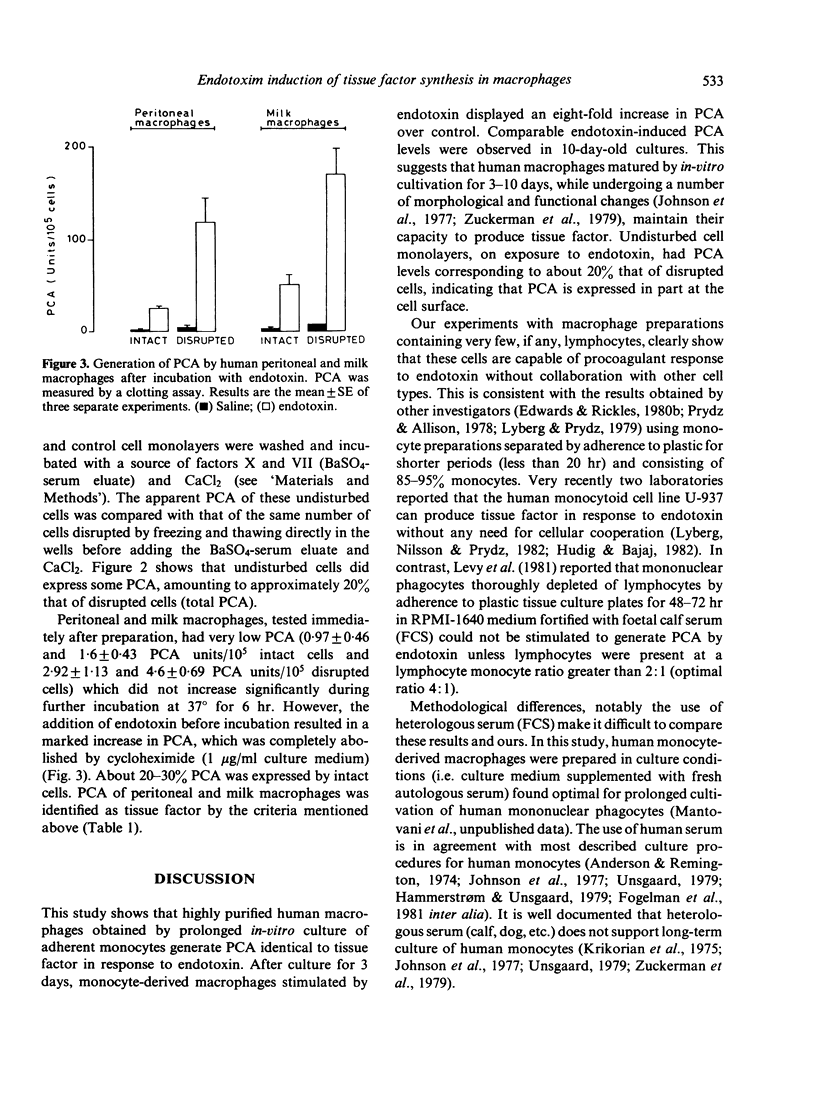
On exposure to endotoxin and other stimuli, human peripheral-blood mononuclear cells generate a potent procoagulant activity (PCA), identified as tissue factor. Although it is now recognized that the monocytes are the source of PCA, the question whether these cells per se are capable of procoagulant response to endotoxin or require lymphocyte collaboration remains unsettled. We have investigated the capacity of highly purified human macrophages from diverse anatomical sites to generate PCA following endotoxin stimulation. Purified (greater than 99%) monocyte-derived macrophages were obtained by prolonged (3-10 days) in-vitro culture of adherent monocytes using medium supplemented with 50% human serum. Purified (greater than 95%) peritoneal and milk macrophages were isolated by adherence to plastic. PCA was measured before and after incubation (4 hr at 37 degrees) with endotoxin (Salmonella enteritidis LPS, W or Escherichia coli O111:B4LPS, W, 1 microgram/ml final concentration) using a one-stage clotting assay and/or a two-stage amidolytic assay. Monocyte-derived macrophages had low baseline PCA (14-19 units/10(5) cells) but, upon exposure to endotoxin, displayed an eight-fold increase in PCA over control. Peritoneal and milk macrophages expressed very low baseline activity (1-5 units/10(5) cells). The latter, however, increased 15-20 times over control following endotoxin stimulation. PCA was identified as tissue factor by biological and immunological criteria. Its generation was completely abolished by cycloheximide. It is concluded that in the human mononuclear phagocyte series the capacity to produce PCA is not restricted to circulating monocytes but is also expressed by macrophages obtained from diverse anatomical sites. These macrophages appear to be autonomous in their procoagulant response to endotoxin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amlie E., Lyberg T., Kaplun A., Hetland O., Prydz H. Thromboplastin activity of mouse peritoneal macrophages. Thromb Res. 1981 Oct 1;24(1-2):61–71. doi: 10.1016/0049-3848(81)90032-3. [DOI] [PubMed] [Google Scholar]
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Colucci M., Curatolo L., Donati M. B., Semeraro N. Cancer cell procoagulant activity: evaluation by an amidolytic assay. Thromb Res. 1980 May 1;18(3-4):589–595. doi: 10.1016/0049-3848(80)90359-x. [DOI] [PubMed] [Google Scholar]
- Cooperstock M. S., Tucker R. P., Baublis J. V. Possible pathogenic role of endotoxin in Reye's syndrome. Lancet. 1975 Jun 7;1(7919):1272–1274. doi: 10.1016/s0140-6736(75)92553-2. [DOI] [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R. The role of human T cells (and T cell products) for monocyte tissue factor generation. J Immunol. 1980 Aug;125(2):606–609. [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Geczy C. L., Hopper K. E. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. II. Lymphokines promote procoagulant activity of macrophages in vitro. J Immunol. 1981 Mar;126(3):1059–1065. [PubMed] [Google Scholar]
- Hammerstrøm J., Unsgaard G. In vitro influence of endotoxin on human mononuclear phagocyte structure and function. 1. Depression of protein synthesis, phagocytosis of Candida albicans and induction of cytostatic activity. Acta Pathol Microbiol Scand C. 1979 Dec;87(6):381–389. [PubMed] [Google Scholar]
- Hiller E., Saal J. G., Riethmüller G. Procoagulant activity of activated monocytes. Haemostasis. 1977;6(6):347–350. doi: 10.1159/000214201. [DOI] [PubMed] [Google Scholar]
- Hudig D., Bajaj S. P. Tissue factor-like activity of the human monocytic tumor cell line U937. Thromb Res. 1982 Aug 1;27(3):321–332. doi: 10.1016/0049-3848(82)90079-2. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian G., Marshall W. H., Simmons S., Stratton F. Counts and characteristics of macrophage precursors in human peripheral blood. Cell Immunol. 1975 Sep;19(1):22–31. doi: 10.1016/0008-8749(75)90288-9. [DOI] [PubMed] [Google Scholar]
- Levy G. A., Edgington T. S. Lymphocyte cooperation is required for amplification of macrophage procoagulant activity. J Exp Med. 1980 May 1;151(5):1232–1244. doi: 10.1084/jem.151.5.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. A., Schwartz B. S., Edgington T. S. The kinetics and metabolic requirements for direct lymphocyte induction of human procoagulant monokines by bacterial lipopolysaccharide. J Immunol. 1981 Jul;127(1):357–363. [PubMed] [Google Scholar]
- Lyberg T., Nilsson K., Prydz H. Synthesis of thromboplastin by U-937 cells. Br J Haematol. 1982 Aug;51(4):631–641. doi: 10.1111/j.1365-2141.1982.tb02827.x. [DOI] [PubMed] [Google Scholar]
- Lyberg T., Prydz H. Lectin stimulation of tissue thromboplastin activity in human monocytes in vitro. Thromb Haemost. 1980 Feb 29;42(5):1574–1579. [PubMed] [Google Scholar]
- Maier R. V., Ulevitch R. J. The response of isolated rabbit hepatic macrophages (H-M macrophage) to lipopolysaccharide (LPS). Circ Shock. 1981;8(2):165–181. [PubMed] [Google Scholar]
- Mantovani A., Dean J. H., Jerrells T. R., Herberman R. B. Augmentation of tumoricidal activity of human monocytes and macrophages by lymphokines. Int J Cancer. 1980 Jun 15;25(6):691–699. doi: 10.1002/ijc.2910250602. [DOI] [PubMed] [Google Scholar]
- Prydz H., Allison A. C. Tissue thromboplastin activity of isolated human monocytes. Thromb Haemost. 1978 Jun 30;39(3):582–591. [PubMed] [Google Scholar]
- Schwartz B. S., Edgington T. S. Immune complex-induced human monocyte procoagulant activity. I. a rapid unidirectional lymphocyte-instructed pathway. J Exp Med. 1981 Sep 1;154(3):892–906. doi: 10.1084/jem.154.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. H., Adams J. G., 3rd, Dreiling B. J. Alpha thalassaemia in adults with sickle-cell trait. Br J Haematol. 1975 May;30(1):31–37. doi: 10.1111/j.1365-2141.1975.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Unsgaard G. Cytotoxicity to tumour cells induced in human monocytes cultured in vitro in the presence of different sera. Acta Pathol Microbiol Scand C. 1979 Apr;87C(2):141–149. [PubMed] [Google Scholar]
- Zanella A., Mantovani A., Mariani M., Silvani C., Peri G., Tedesco F. A modified 'low pH' lignocaine method to isolate human monocytes: a comparison with other separation procedures. J Immunol Methods. 1981;41(3):279–288. doi: 10.1016/0022-1759(81)90191-5. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Ackerman S. K., Douglas S. D. Long-term human peripheral blood monocyte cultures: establishment, metabolism and morphology of primary human monocyte-macrophage cell cultures. Immunology. 1979 Oct;38(2):401–411. [PMC free article] [PubMed] [Google Scholar]


