Abstract
In most eukaryotes, replication origins are composed of long chromosome regions, and the exact sequences required for origin recognition complex (ORC) and minichromosome maintenance (MCM) complex association remain elusive. Here, we show that two stretches of adenine/thymine residues are collectively essential for a fission yeast chromosomal origin. Chromatin immunoprecipitation assays revealed that the ORC subunits are located within a 1 kb region of ori2004. Analyses of deletion derivatives of ori2004 showed that adenine stretches are required for ORC binding in vivo. Synergistic interaction between ORC and adenine stretches was observed. On the other hand, MCM subunits were localized preferentially to a region near the initiation site, which is distant from adenine stretches. This association was dependent on adenine stretches and stimulated by a non-adenine element. Our results suggest that association of multiple ORC molecules with a replication origin is required for efficient MCM loading and origin firing in fission yeast.
Keywords: adenine stretch/chromatin immunoprecipitation/pre-replicative complex/replication origin/Schizosaccharomyces pombe
Introduction
Eukaryotic DNA replication initiates from multiple replication origins distributed on chromosomes. Initiation of DNA replication is tightly regulated to occur with appropriate timing from specific regions. Since fixed regions are used as replication origins in almost all eukaryotic species, each replication origin is likely to contain information required to promote DNA replication (see review by Gilbert, 2001).
Structures of replication origins are well understood in the budding yeast, Saccharomyces cerevisiae. Budding yeast origins isolated as autonomously replicating sequences (ARSs) are composed of modular elements within a 100–200 bp region. A consensus sequence, designated as the ARS consensus sequence (ACS), is essential for origin function (Marahrens and Stillman, 1992). The ACS is the binding consensus for a six subunit origin recognition complex (ORC) (Bell and Stillman, 1992). ORC and two other factors, Cdc6 and Cdt1, are prerequisite for origin binding of the minichromosome maintenance (MCM) complex, consisting of Mcm2–7, resulting in a pre-replicative complex (pre-RC) (Diffley et al., 1994; Cocker et al., 1996; Aparicio et al., 1997). In vivo footprinting experiments have suggested that the MCM complex is loaded proximal to the ORC-binding site (Diffley et al., 1994).
Pre-RC assembly is a crucial step for initiation of DNA replication. ORC-, Cdc6/18-, Cdt1-dependent association of the MCM complex with chromatin prior to initiation is observed in many eukaryotes (Coleman et al., 1996; Ogawa et al., 1999; Nishitani et al., 2000). The MCM is essential for both initiation and elongation of DNA replication, probably functioning as a replicative DNA helicase (Ishimi, 1997). CDK and Cdc7–Dbf4 kinase activities are required for initiation of replication, presumably for activation of MCM helicase (Donaldson et al., 1998; Zou and Stillman, 2000). CDK activity also pre vents re-activation of replication origins by repressing the assembly of pre-RC during the S and G2 phases (see review by Diffley, 2001; Nguyen et al., 2001). Assembly of pre-RC is limited to the G1 phase, and this regulation is crucial for ‘once and only once’ replication in a cell cycle.
In contrast to budding yeast, replication origins in most eukaryotes appear to require longer chromosome regions (see review by Gilbert, 2001). Short essential consensus sequences like the budding yeast ACS have not been found in such origins. For instance, several kilobase regions within the 8 kb region of the human β-globin origin are required for origin activity, when it is transferred to an ectopic locus (Aladjem et al., 1998). Amplification of the Drosophila chorion gene cluster requires a 440 bp ACE3 element located ∼1.5 kb from the amplification origin, and several amplification-enhancing elements (AERs) (Delidakis and Kafatos, 1989). Localization of ORC subunits to replication origins in Drosophila and human has been demonstrated previously (Austin et al., 1999; Ladenburger et al., 2002), and the results overall imply that specific sequences distributed over long stretches are important for origin function in metazoan cells. However, such sequence elements have not actually been identified, partly because methods available to analyze the origin structure are limited in metazoan cells due to their inability to maintain the origin region as an autonomously replicating plasmid.
Fission yeast, Schizosaccharomyces pombe, similar to higher eukaryotes, requires a long chromosome region as a replication origin, and studies with ARS fragments have shown that a several hundred base pair region is required for autonomous replication (Maundrell et al., 1988; Caddle and Calos, 1994; Dubey et al., 1994; Clyne and Kelly, 1995; Okuno et al., 1997). Short essential consensus sequences like the budding yeast ACS have not been found among ARSs. Instead, stretches of asymmetrically placed adenine/thymine residues have been identified as important elements for ARS activity (Zhu et al., 1994; Clyne and Kelly, 1995; Dubey et al., 1996; Kim and Huberman, 1998; Okuno et al., 1999). We have identified three regions, regions I, II and III, essential for ARS activity of ars2004 (Okuno et al., 1999). Regions I and III, 40 and 65 bp, respectively, consist of extensive adenines on one strand (Okuno et al., 1999). As shown by several groups, the fission yeast ORC interacts with adenine stretches in vitro (Kong and DePamphilis, 2001; Lee et al., 2001; Takahashi and Masukata, 2001). The in vivo ORC binding to AT-rich regions has been demonstrated recently by in situ footprinting (Kong and DePamphilis, 2002). In addition, it has been reported that Orc1 and Mcm6 associate with replication origins in vivo (Ogawa et al., 1999). The results thus suggest that fission yeast replication origins contain information to localize the ORC and MCM.
Considering the structural similarity of replication origins in fission yeast and metazoans, the reasons why long chromosomal regions are required for initiation of DNA replication may also be shared. To ascertain the sequence requirements for chromosomal replication origins in fission yeast, we deleted essential elements for ARS activity from the chromosomal ori2004 locus and analyzed the origin activity and association with the ORC and MCM. Two adenine stretches in ori2004 are collectively essential for initiation of replication. Adenine stretches are the ORC-binding sites in vivo. They are prerequisite for association of the MCM complex with a region that appears to coincide with the replication initiation site. The results further raise the possibility that multiple ORC molecules distributed within the long region are required for efficient firing of replication origins in organisms that require long chromosomal regions for initiation.
Results
Requirement of adenine stretches for initiation of chromosome DNA replication
Three essential regions, namely regions I, II and III, of 40, 125 and 65 bp, respectively, have been identified in ars2004 by the ARS assay (Okuno et al., 1999). However, it has not been clear whether these elements are indeed functional on the chromosome. To examine this question, each of the three regions, individually or in combination, was deleted from the chromosomal ori2004 locus. For synchronization of the cell cycle, cdc25-22 cells and derivatives carrying the mutant ori2004 were released from G2/M block, and total genome DNA was isolated at 60, 75 and 90 min. Replication intermediates were analyzed by neutral/neutral two-dimensional gel electrophoresis followed by Southern hybridization with the ori2004 probe. Progression through the cell cycle after release from the block was similar among deletion strains, as monitored by assessing populations of septum- containing cells (data not shown).
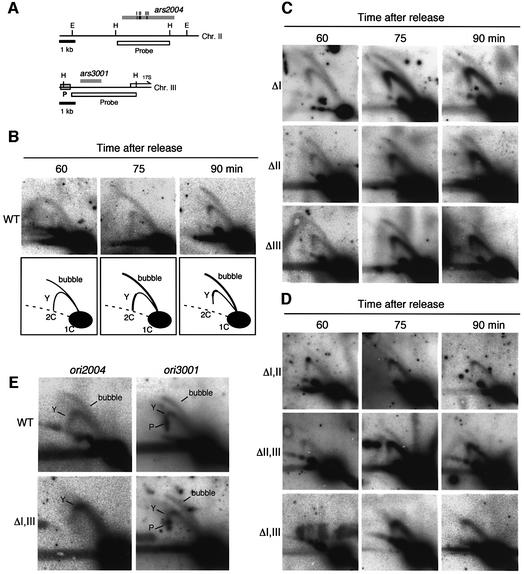
As shown in Figure 1B, two-dimensional gel electrophoresis of the EcoRV-digested DNA from wild-type cells at 60 min yielded a bubble-arc extending from the 1C position to near the 2C position, and a faint Y-arc (Figure 1B, top panel). At later time points, a Y-arc and a bubble-arc at a similar intensity were observed. These results indicate efficient firing of wild-type ori2004 and some extent of passive replication of the ori2004 locus from neighboring origins. In the cells lacking region I (ΔI), the amount of bubble-arc was reduced and the amount of complete Y-arc was increased at 75 and 90 min after release (Figure 1C, top panels), indicating the origin activity of ori2004 to be reduced. In ΔII and ΔIII cells, the bubble-arc was reduced, with a concomitant increment of complete Y-arc as observed in ΔI cells (Figure 1C, middle and bottom panels). These results indicate that regions I, II and III are required for efficient initiation of DNA replication from ori2004.
Fig. 1. Requirement for cis-acting elements for chromosomal ori2004 activity. The cdc25-22 derivatives with an altered ori2004 locus were arrested at 36°C for 4 h and released at 25°C. Total genome DNA was isolated at 60, 75 and 90 min after release and digested with the appropriate restriction enzymes. Replication intermediates (RIs) were analyzed by neutral/neutral two-dimensional gel electrophoresis followed by Southern hybridization. (A) The positions of fragments and probes in ori2004 (upper) and ori3001 (lower) loci are presented. The restriction enzyme sites, EcoRV(E) and HaeIII(H), are indicated. The replication fork pause region at the ori3001 locus identified by Sanchez et al. (1998) is indicated by a gray box. Transcription of the 17S rDNA gene is indicated by an arrow. (B) The results of hybridization of the EcoRV–EcoRV (7.1 kb) ori2004 fragment from cdc25-22 cells at 60, 75 and 90 min after release from G2/M block are presented together with schematic illustrations of bubble- and Y-arcs below the panels. (C) Genomic DNA was prepared from ori2004 mutants lacking region I (ΔI), region II (ΔII) or region III (ΔIII). (D) Genomic DNA was prepared from a strain deleted of both regions I and II (ΔIΔII), II and III (ΔIIΔIII) and I and III (ΔIΔIII). (E) The results of hybridization of HaeIII–HaeIII-digested DNA from cdc25-22 cells containing wild-type ori2004 (WT) and Δregion I–Δregion III (ΔIΔIII), 75 min after release, are presented. The membranes were hybridized with an ori2004 probe (left panels) and then re-hybridized with an ori3001 probe (right panels). The replication pausing signal at the ori3001 locus is indicated as ‘P’.
Since regions I, II and III are not essential for chromosome replication origin activity, we next examined the effect of combined deletion. In ΔIΔII and ΔIIΔIII cells, a strong Y-arc accompanied by a faint bubble-arc was observed at 75 min (Figure 1D, top and middle panels), indicating the initiation from ori2004 to be reduced to a very low level, although residual initiation activity remained. In contrast, in ΔIΔIII cells, no clear bubble-arc was evident (Figure 1D, bottom panels). These results suggest that regions I and III are collectively essential for origin activity of ori2004.
To exclude the possibility that the absence of a bubble-arc in the ΔIΔIII cells might be due to loss of the bubble-shaped intermediates during preparation of genome DNA, the same membranes were analyzed by hybridization with ori2004 and other origin probes. HaeIII-digested genome DNA prepared from wild-type and ΔIΔIII cells at 75 min after release was separated by two-dimensional gel electrophoresis and hybridized with the ori2004 probe, then re-hybridized with the ori3001 probe. As shown in Figure 1E, the wild-type cells yielded both bubble- and Y-arcs with ori2004 and ori3001 probes (Figure 1E, upper panels). In contrast, a bubble-arc was not detected in ΔIΔIII cells with the ori2004 probe, whereas it was present with the ori3001 probe, as in the wild-type cells (Figure 1E, lower panels). From these results, we concluded that regions I and III are collectively essential for activity of ori2004. Region II may not be essential, but is required for efficient origin function.
Cdc18 and Cdt1 promote chromatin association of MCM in G1 phase
Since regions I, II and III are all required for efficient initiation of replication on chromosomes, we examined whether they function in association of ORC or assembly of pre-RC. To examine the association of ORC and MCM with the origin in G1 phase, we used conditions in which MCM is loaded onto chromatin in G1-arrested cells using a temperature-sensitive mutant for the cdc10+ gene. Cdc10 is an essential component of a transcription complex regulating periodic transcription of several genes including cdc18+ and cdt1+, which are required for chromatin association of MCM proteins, and cig2+, which encodes a B-type cyclin acting at the G1–S transition (Kelly et al., 1993; Hofmann and Beach, 1994; Ayte et al., 2001). Thus, cdc10-129 cells arrest in G1 phase without forming pre-RC at the restrictive temperature, and it was expected that ectopic expression of Cdc18 and Cdt1 in cdc10-arrested cells would promote chromatin association of the MCM complex without entrance into S phase. We used pre-synchronization at M phase by a cold-sensitive β-tubulin mutant, nda3-KM311, because it yielded better synchron ization than cdc10-129 alone.
When the cdc10 mutant cells harboring plasmids expressing cdc18+ and cdt1+ genes under control of the inducible nmt1 promoter or vectors alone were released from M phase block and shifted to the restrictive temperature for cdc10-129, the majority were arrested with a 1C DNA content as shown by flow cytometry (Figure 2A). It should be noted that re-replication was not observed with expression of Cdc18 and Cdt1, because we used a weak derivative of the nmt1 promoter. Although cdc18 cloned on a multicopy plasmid suppresses cdc10-129 at the semi-restrictive temperature (Kelly et al., 1993), the cells expressing Cdc18 did not form colonies at 37°C (data not shown). Furthermore, we confirmed that cells expressing Cdc18 and Cdt1 remained in G1 phase by the results showing that the Cdc2 kinase activity measured by phosphorylation of histone H1 was not affected by expression of Cdc18 and Cdt1 in the cdc10-arrested cells (data not shown).
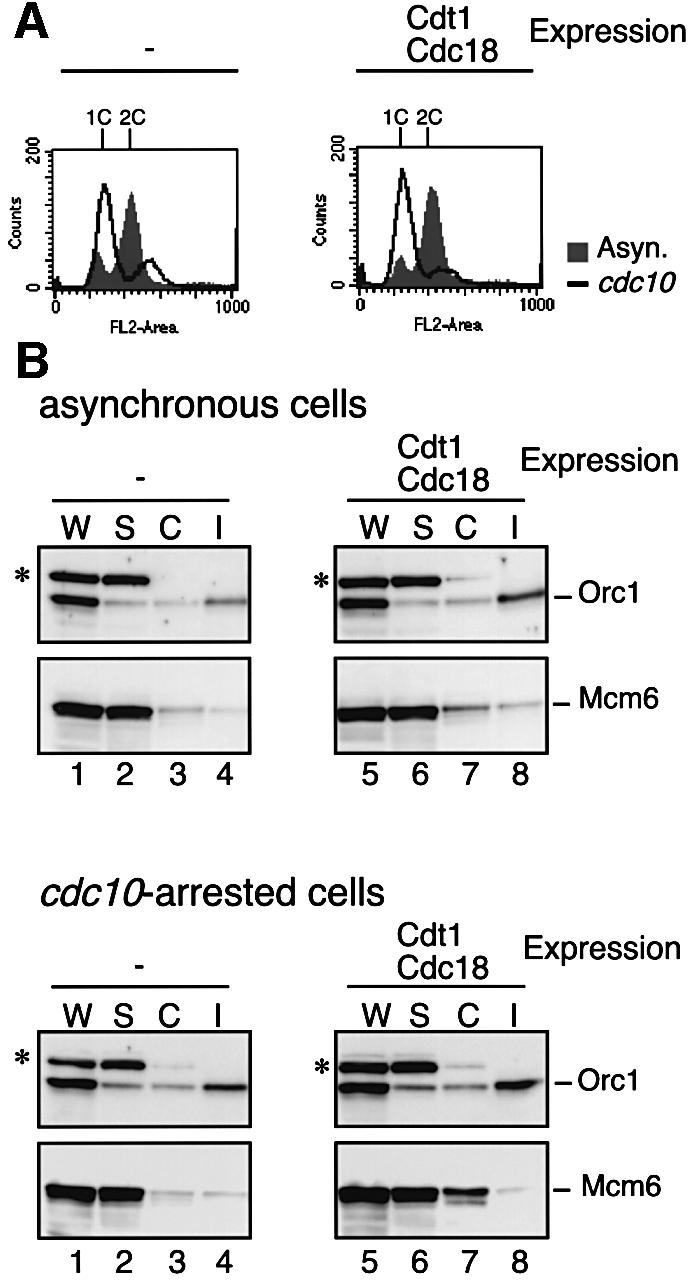
Fig. 2. G1 cell cycle arrest and chromatin association of MCM. TTY44 cells carrying pREP81 and pREP82 vectors (lanes 1–4), or pREP81-cdt1 and pREP82-H6cdc18 (lanes 5–8) were cultured at 28°C in EMM without thiamine, shifted to 20°C for 4 h, and then arrested at G1 phase at 37°C for 3 h. The nda3-KM311 cold-sensitive mutation causing M phase arrest was used for pre-synchronization of cells prior to G1 arrest. (A) The DNA contents of cells were analyzed by FACscan. The positions of 1C and 2C DNA contents are indicated. (B) Proteins in whole-cell extract (W), Triton-soluble fraction (S), chromatin-enriched fraction (C) and insoluble fraction (I) from asynchronous cells (upper panels) and G1-arrested cells (lower panels) were separated by SDS–PAGE and immunoblotted with anti-FLAG (for Orc1) and anti-Mcm6 antibodies. The band marked with an asterisk reacts with anti-FLAG antibody, but is unrelated to Orc1.
To elucidate whether pre-RCs are formed under the above conditions, chromatin association of MCM proteins was analyzed. Cellular proteins (W) were separated into Triton-soluble (S) and -insoluble fractions after cell wall digestion. Chromatin-associated proteins (C) were solu bilized by DNase I digestion and separated from the insoluble fraction (I). Orc1 was recovered mainly in the DNase I-insoluble fraction (I) and relative abundance in the fraction was not affected significantly by the cell cycle or by expression of Cdc18 and Cdt1 (Figure 2B, lanes 4 and 8). Mcm6 was not associated efficiently with chromatin in cdc10-arrested cells harboring vectors alone, as previously reported (Ogawa et al., 1999) (Figure 2B, bottom panel, lane 3). In contrast, about one-third of Mcm6 was found in the chromatin fraction in cdc10-arrested cells expressing Cdc18 and Cdt1 (Figure 2B, bottom panel, lane 7). Mcm2 and Mcm7, other subunits of the MCM complex, also accumulated in the chromatin-enriched fraction (data not shown). Such accumulation of MCM subunits was not observed in asynchronous cells with expression of Cdc18 and Cdt1 (Figure 2B; data not shown), excluding the possibility that MCM is loaded aberrantly onto the chromatin. These results indicate that Cdc18 and Cdt1 expressed in G1-arrested cells promote chromatin association of the MCM complex. Below, we designate this condition as G1 phase.
ChIP scanning
Since regions required for initiation of DNA replication are distributed over ∼1 kb, it is necessary to examine the localization of the pre-RC components within this stretch. For this purpose, we developed a chromatin immunoprecipitation (ChIP) scanning method as described below. DNA fragments associated with ORC or MCM were recovered by immunoprecipitation after in vivo cross-linking with formaldehyde (ChIP method). Recovery of specific regions within the origin was determined by PCR amplification from the immunoprecipitated and total cellular DNA after serial dilution. To minimize the error of PCR amplification, a linear range of PCR products amplified from eight serial dilutions was used for quantitation. For scanning the recovery of DNA fragments along the origin locus, 11 primer sets for the ori2004 locus and a primer set for the ori3002 locus as an internal control were designed.
ORC associates with adenine stretches in regions I and III
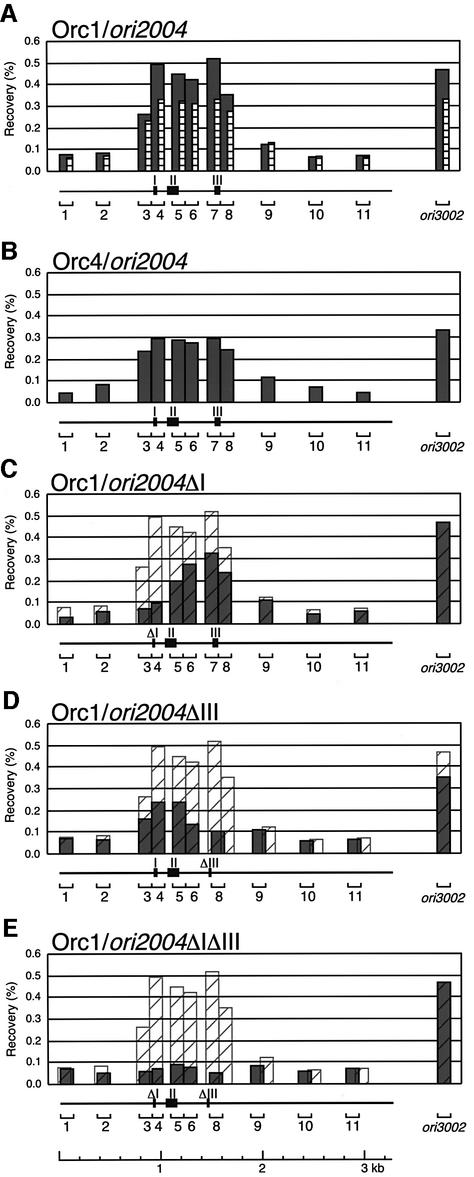
Using the G1 cell extract of TTY44 (h– cdc10-129 nda3-KM311 ura4-D18 leu1-32 orc1-5FLAG) cells expressing Cdc18 and Cdt1 from plasmids, we first examined the localization of ORC subunits. By immunoprecipitation with Orc1, segments within ori2004, ori3002, used as an internal control (see below), and ori1 (data not shown) were amplified preferentially compared with the non-ARS region, suggesting localization of Orc1 at replication origins on fission yeast chromosomes. Figure 3A shows representative results of ChIP scanning for the ori2004 locus from two independent immunoprecipitations. The relative recovery of each segment in ori2004 compared with ori3002, an internal control, was similar in two experiments, although recovery of DNA differed slightly, supporting the reproducibility of the results. Segments 3–8 of ori2004 were recovered by Orc1 immunoprecipitation (Orc1-IP) at a level several times greater than in the surrounding regions (Figure 3A). Furthermore, distribution of Orc4-immunoprecipitated DNA at this locus was similar to that of Orc1-immunoprecipitated DNA (Figure 3B). These results suggest that the fission yeast ORC associates with the region containing regions I, II and III of ori2004.
Fig. 3. Effect of deletion of adenine stretches on Orc1 localization. TTY44 derivatives carrying the wild-type ori2004 locus (A and B), ori2004ΔI (C), ori2004ΔIII (D) or ori2004ΔIΔIII (E), harboring pREP81-cdt1 and pREP82-H6cdc18, were arrested in G1 phase as described in Figure 2. Total cellular DNA and DNA immunoprecipitated with anti-FLAG for FLAG-Orc1 (A, C, D and E) or anti-Orc4 (B) antibodies, after serial dilution as templates for PCR amplification. Primers used were 11 sets for the ori2004 locus, and one for the ori3002 locus as an internal control. PCR products were analyzed as described in Materials and methods. The recovery of immunoprecipitated against total DNA calculated using a linear range of PCR amplification is presented in histogram form. Shaded and striped histograms in (A) show the results of two independent Orc1-IPs. Hatched histograms in (C–E) show superimposed wild-type ori2004 DNA immunoprecipitated with Orc1. The positions of regions I, II and III in ori2004 are indicated by filled boxes.
It has been shown that the ORC immunoprecipitate specifically interacts in vitro with adenine stretches in regions I and III of ars2004 (Takahashi and Masukata, 2001). In order to determine whether ORC interacts with these regions in vivo, the effects of deletion of regions I and/or III on the ORC–ori2004 association were examined. In ΔI cells, the recovery of DNA fragments around region I was greatly reduced (Figure 3C). Reciprocally, the recovery of DNA fragments around region III was reduced in ΔIII cells (Figure 3D). Furthermore, when both adenine stretches were deleted, no specific region within ori2004 was recovered by Orc1-IP, indicating that Orc1 association with ori2004 depends on adenine stretches in regions I and III (Figure 3E). A similar amount of the ori3002 locus, an internal control, was recovered, except in Figure 3D, where recovery by immunoprecipitation was slightly lower than in the other experiments. These results strongly suggest that the fission yeast ORC binds to adenine stretches in regions I and III in vivo. Interestingly, deletion of either adenine stretch reduced recovery of the remaining adenine stretch region (Figure 3C and D). These results suggest that ORC synergistically interacts with two adenine stretches in ori2004.
MCM is localized to a region distinct from the adenine stretches
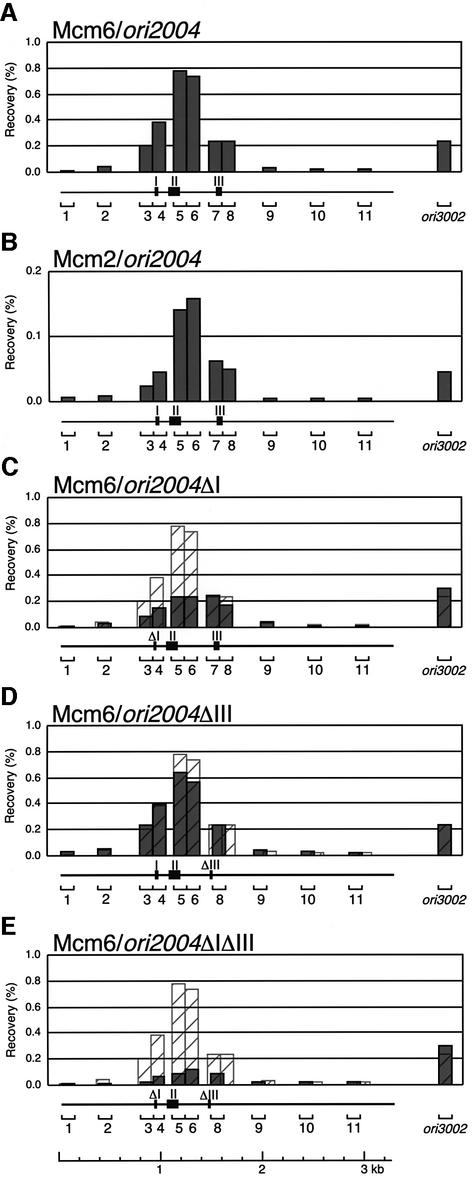
Next, association of MCM subunits with ori2004 was examined by ChIP scanning. In contrast to the localization of ORC subunits, DNA fragments recovered by Mcm6- and Mcm2-IP showed an apparent peak (Figure 4). Segments 5 and 6 were recovered preferentially by Mcm6-IP at 2- or 3-fold higher levels than segments 3 and 4 around region I, and segments 7 and 8 around region III (Figure 4A). The distribution of DNA fragments recovered by Mcm2-IP was very similar to that with Mcm6-IP (Figure 4B), suggesting that the MCM complex preferentially associates with a region in or near segments 5 and 6 in the ori2004 locus. Taking these results together with those in Figure 3, MCM appears to associate with a region distinct from ORC association sites in ori2004.
Fig. 4. Effects of deletion of adenine stretches on Mcm6 localization. ChIP scanning experiments were carried out as described in Figure 3, except that DNA was immunoprecipitated with anti-Mcm6 (A, C, D and E) or anti-Mcm2 (B) antibodies. The recovery of the immunopre cipitated DNA relative to total cellular DNA is shown by histograms with relevance to the positions of primer sets. The hatched histograms in (C–E) show superimposed recovery of wild-type ori2004 DNA immunoprecipitated with anti-Mcm6.
Two adenine stretches in ori2004 are required for efficient association of MCM
Because association of ORC with the replication origin is prerequisite for loading of MCM (Aparicio et al., 1997), we examined how two adenine stretches contribute to MCM association. By deleting region I, recovery of segments 5 and 6 by Mcm6-IP was reduced to about one-third of the value with the wild-type strain (Figure 4C). Deletion of region III reduced recovery of segments 5 and 6 by about a quarter (Figure 4D). By combination of two deletions (ΔIΔIII, Figure 4E), recovery of the segments within the ori2004 locus was drastically reduced to about one-tenth of the wild type. Recovery at the ori3002 locus was not affected significantly by these deletions, indicating that the loss of MCM association was specific to ori2004. These results confirmed that ORC association with regions I and III is collectively essential for loading of MCM onto ori2004. The results also show that both adenine stretches at the ori2004 locus are required for efficient association of MCM. However, their contribution to MCM loading appears to differ, the MCM association being more severely impaired by deletion of region I.
Region II functions for association of MCM with ori2004
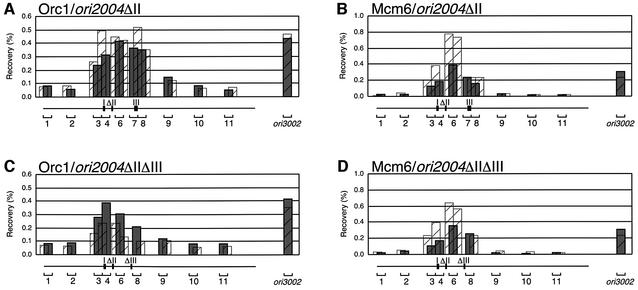
Region II, which was found not to interact with ORC in our previous in vitro study (Takahashi and Masukata, 2001), nevertheless is important for the origin function on the chromosome. To elucidate its roles, we examined effects of its deletion on association of ORC and MCM using the ChIP scanning assay. As shown in Figure 5, while a lack of region II only slightly reduced the Orc1 association, recovery of DNA fragments of segment 6 by Mcm6-IP was reduced by about half (Figure 5A and B). These results suggest that region II is required for efficient association of MCM with ori2004.
Fig. 5. Effects of deletion of region II on Orc1 and Mcm6 localization at the ori2004 locus. ChIP scanning was performed as described in Figure 3 using ori2004ΔII (A and B) and ori2004ΔIIΔIII (C and D). DNA associated with Orc1 (A and C) and Mcm6 (B and D) was recovered by immunoprecipitation as shown in histogram form relative to total cellular DNA. Hatched histograms superimpose recovery of Orc1-IP DNA from wild-type ori2004 (A) and that from ori2004ΔIII (C), or recovery of Mcm6-IP DNA from wild-type ori2004 (B) and that from ori2004ΔIII (D).
To confirm the above results, the effects of region II deletion were examined in the ΔIII background, which eliminated the contribution of region III to ORC and MCM association. As shown in Figure 5C, association of Orc1 around region I was not reduced further in ΔIIΔIII cells compared with ΔIII cells (shown by hatched histograms). On the other hand, recovery of origin DNA by Mcm6-IP in ΔIIΔIII cells was about a half of that in ΔIII cells (Figure 5D). These results strongly suggest that region II functions in the association of MCM.
Localization of the MCM complex is not determined by the orientation of the adenine stretch
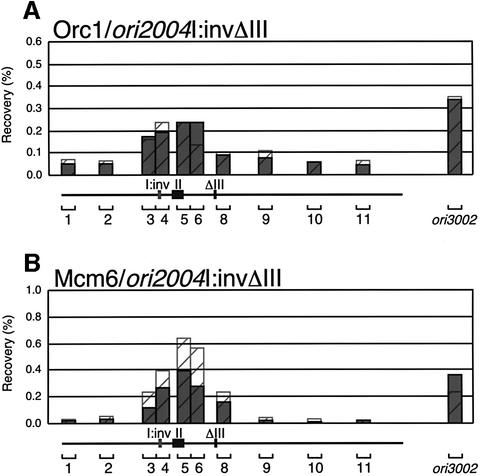
Because the MCM-binding region lies 3′ to the adenine-rich strands of regions I and III, the direction of adenine stretches, which would specify the polarity of the ORC complex, may determine the MCM localization site. To test this possibility, we made a strain carrying an inverted region I and analyzed the localization of ORC and MCM. Since two adenine stretches in ori2004 had a redundant role for ORC binding, region III was deleted to eliminate its effect. In the plasmid ARS assay, a derivative of the 3.2 kb ars2004ΔIII plasmid carrying inverted region I formed transformants, suggesting that the inversion did not impair the ARS activity (data not shown). As shown in Figure 6, association of Orc1 in the region I-inverted cells (I:inv ΔIII) was essentially the same as in ΔIII cells. While recovery of segment 5 by Mcm6-IP in the I:inv ΔIII cells was reduced by about half, the peak position of Mcm6 localization was not significantly affected. These results suggest that the MCM localization site is not determined simply by the direction of the adenine stretch. They also imply that the context between the adenine stretch and the other regions of ori2004 might be important for MCM loading.
Fig. 6. Effects of inversion of region I on MCM localization. The results of ChIP scanning with a strain carrying an inverted region I for the ori2004 locus are presented. Recovery of Orc1-IP DNA (A) and Mcm6-IP DNA (B) from the strain carrying the inverted region I in the ΔIII background is shown in histogram form. Hatched histograms in (A) and (B) superimpose recoveries from the ΔIII strain carrying region I in the natural orientation.
Discussion
The nucleotide sequences required for initiation of chromosome DNA replication have not been identified in most eukaryotes except for budding yeast. Here, we demonstrated that replication origin activity on the fission yeast chromosome depends on specific sequence elements. The 40 and 65 bp adenine stretches in ori2004 are collectively essential for the replication origin function. The ChIP assay with deletions of adenine stretches shows that the essential role of the adenine stretches is association with ORC. On the other hand, MCM association occurs in a region distinct from the adenine stretches. Efficient loading of MCM requires multiple adenine stretches, which are presumably associated with multiple ORC molecules.
Multiple adenine stretches are required for efficient firing of chromosomal replication origins
Several groups have shown that asymmetrically aligned adenine/thymine stretches are essential for ARS activity in fission yeast (Zhu et al., 1994; Clyne and Kelly, 1995; Dubey et al., 1996; Kim and Huberman, 1998; Okuno et al., 1999). However, the dependence of the ARS activity on each adenine stretch becomes less clear in long chromosome fragments cloned on plasmids (Okuno et al., 1999). Here, we showed that both adenine stretches in ori2004 are collectively important for efficient firing of the origin. Our results suggest that multiple adenine stretches, which are more widely distributed in some origins, are redundant to some extent, but collectively are essential components of the replication origin in fission yeast.
ORC binds to the adenine stretches
The results of ChIP experiments demonstrated that Orc1 and Orc4 are associated preferentially with replication origins such as ori2004, ori3002 and ori1. We have carried out detailed ChIP scanning of the ori2004 locus, partly because it is the most efficient origin both on the chromosome and on plasmids, and because deletion mutants of the chromosome locus are available. The results of ChIP scanning from independent Orc1-IPs yielded almost identical distributions of Orc1-associated segments (Figure 3A), although recovery of DNA differed slightly between experiments. Moreover, because the distributions of DNA immunoprecipitated with Orc1 and Orc4 were almost the same, localization of the ORC complex thereby would be detected. The requirement for regions I and III for the ORC association indicates that ORC binds to these regions. This conclusion is consistent with previous in vitro observations that ORC specifically interacts with regions I and III as well as with synthetic adenine stretches (Takahashi and Masukata, 2001). It has been shown that the N-terminal region of Orc4, which contains multiple AT-hook motifs, is responsible for ORC binding to the AT-rich tracts in ars3002 and ars1 (Chuang and Kelly, 1999; Kong and DePamphilis, 2001; Lee et al., 2001). In addition, a recent report has shown that in vitro binding sites of Orc4 are occupied in vivo, suggesting that Orc4 binds to AT-rich sites in vivo (Kong and DePamphilis, 2002). Thus, interaction of Orc4 with adenine stretches might be responsible for preferential association of ORC with replication origins in vivo.
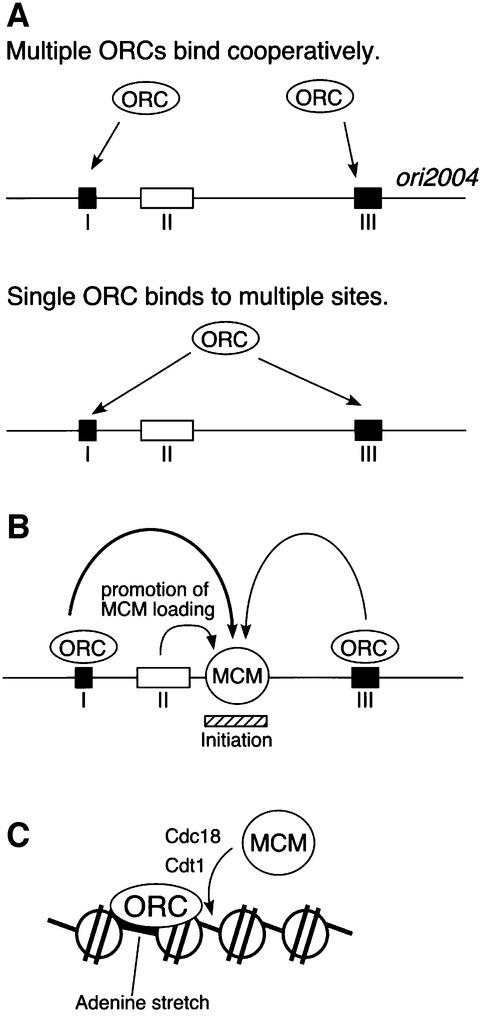
The finding that ORC is localized at distantly located adenine stretches might be interpreted as association of ORC with one of alternative sites in different cells. For instance, ORC binds to region I of ori2004 in one cell, while it associates with region III in another cell. However, this might not be the case, because the results of ChIP experiments with ΔI or ΔIII cells showed that ORC interacts synergistically with two adenine stretches (Figure 3). This would allow us to raise the following possibilities. First, multiple ORC molecules cooperatively interact with adenine stretches in the origin (Figure 7A). Alternatively, a single ORC molecule interacts with multiple adenine stretches (Figure 7A). We have shown that ORC immunoprecipitates interact with a 40 bp adenine/thymine stretch, which corresponds to region I of ori2004 (Takahashi and Masukata, 2001). Thus, it seems more likely to us that at least two ORC molecules may bind cooperatively to regions I and III of ori2004, although we cannot exclude the latter possibility.
Fig. 7. Model of the structure of pre-RC in fission yeast. (A) Two alternative models for ORC association with ori2004 are shown. Adenine stretches indicated by black boxes labeled with I and III are the ORC-binding sites. (B) Adenine stretches are collectively essential for loading of MCM onto a region distant from the adenine stretches. The non-adenine element shown by an open box (II) stimulates the MCM loading reaction. The initiation site mapped by two-dimensional gel electrophoresis is shown by a hatched box. (C) Regular nucleosomes appear to be formed within ori2004. ORC might associate with the MCM loading region across nucleosomes to load the MCM complex. Although multiple ORC and MCM molecules might be involved, one molecule of each is shown for illustration.
Non-adenine element enhances pre-RC formation
The results of two-dimensional gel analysis showed that region II, which is composed of non-adenine sequences, indeed is involved in chromosomal origin activity (Figure 1). However, residual firing was detected in ΔIΔII and ΔIIΔIII cells but not in ΔIΔIII cells, suggesting that region II is not essential. Consistent with this notion, experiments with ARS plasmid showed that region I or III fragments can functionally replace the two other regions, while the region II fragment cannot (Okuno et al., 1999). The results of ChIP experiments suggest that region II is important for MCM loading. Region II may be a stimulating element for pre-RC formation (Figure 7B).
It has been reported that a replication enhancer element derived from the ura4+ locus origin cluster increases the stability of other ARS fragments in a distance- and orientation-independent manner, although it does not show ARS activity itself (Kim and Huberman, 1999). Because there is no significant sequence homology between region II and the enhancer element from the ura4+ locus replication origins, it is not known whether there is any sequence specificity. However, the results of linker scanning analysis of region II suggested that the included sequence is crucial for function (Okuno et al., 1999). Region II may provide binding sites for certain DNA-binding proteins, possibly for factors involved in pre-RC formation, such as Cdt1, Cdc18 and the Noc3 homolog (Zhang et al., 2002). Alternatively, proteins involved in transcription might bind, since most fission yeast origins including ori2004 are located at the intergenic promoter region upstream of two genes (Gomez and Antequera, 1999). Such proteins binding to region II might modulate chromatin structure at the origin locus and enhance accessibility of pre-RC components to the origin locus.
Localization of MCM subunits within the replication origins
ChIP experiments showed that two MCM subunits, Mcm2 and Mcm6, are localized preferentially to the region distant from the adenine stretches, suggesting distinct localization of the MCM complex and the ORC (Figure 4). Since MCM is considered to function as a DNA helicase for initiation of DNA replication, such a specific localization of the MCM complex might be involved in determination of the initiation site of DNA synthesis. Consistent with this notion, the initiation site at ori2004 mapped by two-dimensional gel analyses appears to coincide with the peak of MCM distribution (Okuno et al., 1997). In addition, recent results have suggested that the initiation site and pre-RC site are almost the same in ars3001 (Kong and DePamphilis, 2002). Thus, initiation of DNA synthesis may occur near the region where the MCM complex associates in the origin.
If the MCM is loaded onto the region where it is localized by ChIP analysis, how can it be loaded at the region distant from the adenine stretch where ORC binds? Although MCM is localized near region II, which appears to stimulate MCM loading, the MCM-binding site was not affected much by deletion of region II. On the other hand, the fact that deletion of region I has a more severe effect on MCM loading than region III deletion (Figure 4) implies that the ORC bound to region I is more proficient at MCM loading. However, the direction of the ORC binding sequence is not important for localization of MCM, because inversion of the adenine stretch of region I has little effect on the MCM-binding site (Figure 6). Since ORC, Cdc18 and Cdt1 may interact with MCM during pre-RC formation, it is possible that MCM is loaded onto a region that becomes proximal to the adenine stretches in the chromatin structure (Figure 7C). Consistent with this idea, micrococcal nuclease digestion of chromatin yields regular nucleosome ladders within ori2004, while region I appears to be nucleosome free (Y.Yamada and H.Masukata, unpublished results). At present, we cannot exclude other possibilities, e.g. that sequence- or structure-specific binding of pre-RC components determines MCM localization.
Origin structure in higher eukaryotes
This study demonstrated that two ORC-binding sites in ori2004 are required for efficient ORC binding, MCM loading and, consequently, for efficient firing. Synergistic requirement of adenine stretches for ORC binding suggests cooperative binding of multiple ORC molecules. Because many fission yeast origins contain multiple adenine stretches that are important for ARS activity, a requirement for multiple ORC-binding sites in an origin seems to be a general feature in fission yeast. Thus, long chromosome regions in fission yeast replication origins are, at least in part, required for efficient ORC association. However, MCM localization in a narrow region may promote initiation of DNA replication from a limited region.
In metazoans, replication origins generally are also composed of long chromosome regions containing multiple redundant elements, except for some small replication origins such as the lamin B2 origin. Thus, binding of multiple ORC molecules to the origin might be required for origin function. One piece of evidence supporting this possibility is that the Drosophila ORC (DmORC) binds to four DNA fragments derived from the chorion gene amplification origin (Austin et al., 1999). DmORC binds to three fragments of ACE3 and a fragment of AER-d. Interestingly, ACE3, which is required for efficient amplification of the locus, is located ∼1.5 kb away from AER-d, where the majority of initiation occurs during amplification. It is possible that DmORC bound to the ACE3 contributes to the initiation from AER-d by loading of MCM onto the AER-d. Long chromosome regions might be required for efficient ORC binding in most eukaryotes.
This work shows that fission yeast replication origins are a useful model system for analysis of functions of redundant and complex elements in eukaryotic replication origins. It will be extremely interesting to understand how multiple ORC binding acts to load MCM onto a distinct site by developing an in vitro system, in combination with in vivo study of the effects of chromatin structures on initiation of replication.
Materials and methods
Strains and media
The S.pombe strains used are listed in Table I. TTY44 was made by standard genetic methods as described earlier (Alfa et al., 1993). They were cultured in a complete medium, YE (0.5% yeast extract, 3% glucose), and an Edinburgh minimal medium, EMM (Moreno et al., 1991). TTY44, 78, 81, 84, 95, 97, EOY75 and 79 cells grown at 28°C were first incubated at 20°C for 4 h for arrest in M phase and then shifted to 37°C for 3 h for arrest in G1 phase. Media containing 2% agar were used for plating. Transformation of S.pombe was performed by electroporation (Hood and Stachow, 1990).
Table I. Schizosaccharomyces pombe strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| TTY15 | h– orp1::5FLAG-orp1 | Our stocka |
| HM288 | h+/h– ade6-M216/ ade6-M210 ura4-D18/ura4-D18 leu1-32/leu1-32 | Our stock |
| EOY7 | h+ ade6-M210 ura4-D18 leu1-32 ori2004::ura4+ | This work |
| EOY68 | h+ cdc25-22 ori2004ΔI | This work |
| EOY70 | h+ cdc25-22 ori2004ΔII | This work |
| EOY72 | h+ cdc25-22 ori2004ΔIII | This work |
| EOY85 | h– cdc25-22 ori2004ΔIΔII | This work |
| EOY87 | h+ cdc25-22 ori2004ΔIIΔIII | This work |
| EOY90 | h– cdc25-22 ori2004ΔIΔIII | This work |
| TTY44 | h– ura4-D18 leu1-32 nda3-KM311 cdc10-129 orp1::5FLAG-orp1 | This work |
| TTY78 | h+ ura4-D18 leu1-32 nda3-KM311 cdc10-129 orp1::5FLAG-orp1 ori2004ΔI | This work |
| EOY75 | h– ura4-D18 leu1-32 nda3-KM311 cdc10-129 orp1::5FLAG-orp1 ori2004ΔII | This work |
| TTY81 | h– ura4-D18 leu1-32 nda3-KM311 cdc10-129 orp1::5FLAG-orp1 ori2004ΔIII | This work |
| TTY84 | h– ura4-D18 leu1-32 nda3-KM311 cdc10-129 orp1::5FLAG-orp1 ori2004ΔIΔIII | This work |
| EOY79 | h+ ura4-D18 leu1-32 nda3-KM311 cdc10-129 orp1::5FLAG-orp1 ori2004ΔIIΔIII | This work |
| TTY95 | h– ura4-D18 leu1-32 nda3-KM311 cdc10-129 orp1::5FLAG-orp1 ori2004I:inv | This work |
| TTY97 | h– ura4-D18 leu1-32 nda3-KM311 cdc10-129 orp1::5FLAG-orp1 ori2004I:inv,ΔIII | This work |
Construction of ori2004 mutant strains
Construction of ori2004 mutant strains was as described below. The 5′ and 3′ regions of ars2004 in pXN289 (Okuno et al., 1997) were PCR amplified with HindIII site using primers 5′-GTAAAACGACGGCCA GT-3′, 5′-TTTAAGCTTCACGGCATCTTTCTTC-3′, and 5′-ACAAAG CTTTCTTCTGGAACTGCTG-3′, 5′-GGAAACAGCTATGACCATG-3′, digested with NotI–HindIII and HindIII–XbaI, respectively, and cloned into NotI–XbaI sites of a derivative of pBluescriptII SK+ lacking the HindIII site. Then, the 1.7 kb HindIII fragment of ura4+ was inserted into the HindIII site. The resulting pARS2004Δ940 was digested with KpnI and XbaI, and used for transformation of HM288. Ura+ transformants were selected, and integration of the Δars2004 fragment was confirmed by Southern hybridization. Haploid strain EOY7 carrying Δori2004:: ura4+ was obtained by tetrad dissection of the resulting strain. The ori2004ΔI, ΔII, ΔIII, ΔIΔII, ΔIIΔIII, ΔIΔIII, I:inv and I:invΔIII strains were obtained by transformation of EOY7 with KpnI–XbaI-digested pARS2004ΔI, ΔII, ΔIII, ΔIΔII, ΔIIΔIII, ΔIΔIII (Okuno et al., 1999), p2004I:inv and p2004I:invΔIII (see below) plasmids, and ura– transformants were selected on 5-fluoro-orotic acid (5-FOA)-containing plates. In every case, integration was confirmed by Southern hybridization. p2004I:inv and p2004I:invΔIII were constructed as described below. The 3 kb KpnI–XbaI fragment of pARS2004ΔI was cloned into pBluescriptII SK+. Region I oligonucleotides 5′-GTT AAAAAAAATTAAAAATTAACAAAAAAAAAAAAAAAAAAACC TGCA-3′ and 5′-GGTTTTTTTTTTTTTTTTTTTGTTAATTTTTAATT TTTTTTAACTGCA-3′ were annealed, and inserted into the Sse8387I site in the plasmid, resulting in p2004I:inv. For construction of p2004I:invΔIII, a 0.5 kb BamHI–HindIII fragment of pARS2004ΔIII was cloned into BamHI–HindIII sites of p2004I:inv. EOY68, 70, 72, 75, 79, 85, 87, 90, TTY78, 81, 84, 95 and 97 were obtained by a standard genetic cross of the ori2004 mutant strains with cdc25-22 (Fantes, 1979), cdc10-129 (Nurse et al., 1976) and/or nda3-KM311 (Hiraoka et al., 1984) strains.
Construction of plasmids
Construction of the Cdc18 expression vector pREP82-H6cdc18 was as described below. The N-terminal region of Cdc18 on pREP3x-cdc18 (Kelly et al., 1993) was PCR amplified with a His tag and the NdeI site using primers 5′-AAGGATCCATATGCACCACCACCACCACCACT GTGAAACTCCAATAGGT-3′ and 5′-TGTGGGTGTTTGGAAATG-3′, and the products were digested with NdeI and PstI. The resulting NdeI–PstI fragment containing the N-terminal region of Cdc18 together with the PstI–BamHI fragment containing the C-terminal region of cdc18 derived from pREP3x-cdc18 was inserted into NdeI–BamHI sites of pREP82 (Basi et al., 1993), resulting in pREP82-H6cdc18. H6Cdc18 is fully functional because ectopic expression of H6Cdc18 complemented cdc18-K46 mutation at 37°C. Construction of the Cdt1 expression vector pREP81-cdt1 will be described elsewhere.
Preparation of chromatin-enriched fractions and immunoblotting
Chromatin-enriched fractions were prepared as described previously, with some modification (Ogawa et al., 1999). Spheroplasts resuspended in HNG buffer [10 mM HEPES–NaOH pH 7.2, 50 mM NaAc, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 µg/ml leupeptin, 50 µg/ml TLCK, 2 µg/ml aprotinin] at a concentration of 108 cells/ml were lysed by addition of Triton X-100 at a final concentration of 1% for 5 min on ice, and the insoluble fraction was recovered by centrifugation for 15 min at 20 000 g, washed and incubated at 20°C for 20 min in HNG buffer containing 150 mM NaCl, 1.5 mM Mg(Ac)2 and 2800 U/ml DNase I (TaKaRa). The DNase I-soluble fraction was separated from the insoluble fraction by centrifugation for 15 min at 20 000 g. Immunoblotting was performed as described previously (Takahashi and Masukata, 2001), with affinity-purified rabbit anti-Mcm6, anti-Mcm2 antibodies or mouse anti-FLAG M2 antibody (Kodak) at 1:1000, 1:1000 and 1:3000 dilutions, respectively.
ChIP
ChIP was accomplished as described previously (Ogawa et al., 1999).
PCR amplification with ampli-Taq Gold (Perkin-Elmer) was performed in 30 µl of PCR buffer containing 1.5 mM MgCl2 (Perkin-Elmer) with each set of primers, and immunoprecipitated DNA or total DNA. Immunoprecipitated DNA was used at dilutions of 1/1500–1/192 000, while total DNA was at dilutions of 1/800 000–1/102 400 000 as templates. The nucleotide sequences of the primers used are listed in Table II. All were used at a concentration of 0.2 µM. PCR products were separated in 3% agarose gels and visualized with 0.5 µg/ml ethidium bromide. The gel images obtained with a charge-coupled device camera (LAS1000+; Fujifilm) were processed using Image Gauge software (Fujifilm). Recovery (%) was calculated from the linear range of PCR products with the equation:
Table II. Sequences of the primers used in this study.
| Name | Sequence (5′–3′) | Length of PCR fragment |
|---|---|---|
| ori2004 locus | ||
| 2004-1F | CCCTCACTAAAGGGATCATCCTC | |
| 2004-1R | CCCTCCAAACCCTCCAAACCTCGC | 140 |
| 2004-2F | GGATGCTTTCGAAATCCCTCGTG | |
| 2004-2R | GGTGAGGTGGTAAAACGTACGC | 143 |
| 2004-3F | GTTTGTCTCCGATAACGGGGG | |
| 2004-3R | CACGGCATCTTTCTTCACGA | 140 |
| 2004-4F | TCGTGAAGAAAGATGCCGTG | |
| 2004-4R | GTGTGGTGTGTACTGAGTC | 143 |
| 2004-5F | GCTAATTGAATGGAATGATTTGC | |
| 2004-5R | GGGATTACGGATCCGAAAACTACCC | 145 |
| 2004-6F | GGGTAGTTTTCGGATCCGTAATCCC | |
| 2004-6R | CATACCAACCCTTACAAC | 144 |
| 2004-7F | GAAACTTGTATATTATTTCTGCGTATAACC | |
| 2004-7R | GAGTAATTAGCAATGTATGAATAGATCCAGCAGTTCC | 142 |
| 2004-8F | CGTAGGTCTTCTGGAACTGCTGG | |
| 2004-8R | GAAGCTAAATCGTTGCGTG | 146 |
| 2004-9F | GTATCGTCTTGCTCGGTTTATTCCAGACCC | |
| 2004-9R | CCTAGTGCCACACTTCCACC | 140 |
| 2004-10F | GAGCGTGATAAAAACGTGACGGG | |
| 2004-10R | CAACCCCATAACGATCAGTG | 141 |
| 2004-11F | CTCTACGCTTGACTGTACTCTCTC | |
| 2004-11R | GGGATGTTCCTACTCTTTGTTC | 143 |
| |
|
|
| ori3002 locus | ||
| 3002-F | CCTGTTGAAATATGTATTTGGCGC | |
| 3002-R | GATAGCTTTTGGATAAGTTATGACTTTTACG | 141 |
Recovery (%) = immunoprecipitated DNA/total DNA × 100
Neutral/neutral two-dimensional gel electrophoresis
Neutral/neutral two-demensional gel electrophoresis was performed as described previously (Okuno et al., 1997).
Acknowledgments
Acknowledgements
We thank T.Tsurimoto and T.Nakagawa for critical reading of the manuscript. This study was supported in part by a grant-in-aid from the Ministry of Education, Science, Technology, Sports and Culture, Japan, to H.M.
References
- Aladjem M.I., Rodewald,L.W., Kolman,J.L. and Wahl,G.M. (1998) Genetic dissection of a mammalian replicator in the human β-globin locus. Science, 281, 1005–1009. [DOI] [PubMed] [Google Scholar]
- Alfa C., Fantes,P., Hyams,J., Mcleod,M. and Warbrick,E. (1993) Experiments with Fission Yeast. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Aparicio O.M., Weinstein,D.M. and Bell,S.P. (1997) Components and dynamics of DNA replication complexes in S.cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell, 91, 59–69. [DOI] [PubMed] [Google Scholar]
- Austin R.J., Orr-Weaver,T.L. and Bell,S.P. (1999) Drosophila ORC specifically binds to ACE3, an origin of DNA replication control element. Genes Dev., 13, 2639–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayte J., Schweitzer,C., Zarzov,P., Nurse,P. and DeCaprio,J.A. (2001) Feedback regulation of the MBF transcription factor by cyclin Cig2. Nat. Cell Biol., 3, 1043–1050. [DOI] [PubMed] [Google Scholar]
- Basi G., Schmid,E. and Maundrell,K. (1993) TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene, 123, 131–136. [DOI] [PubMed] [Google Scholar]
- Bell S.P. and Stillman,B. (1992) ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature, 357, 128–134. [DOI] [PubMed] [Google Scholar]
- Caddle S. and Calos,M.P. (1994) Specific initiation at an origin of replication from Schizosaccharomyces pombe. Mol. Cell. Biol., 14, 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang R.Y. and Kelly,T.J. (1999) The fission yeast homologue of Orc4p binds to replication origin DNA via multiple AT-hooks. Proc. Natl Acad. Sci. USA, 96, 2656–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne R.K. and Kelly,T.J. (1995) Genetic analysis of an ARS element from the fission yeast Schizosaccharomyces pombe. EMBO J., 14, 6348–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker J.H., Piatti,S., Santocanale,C., Nasmyth,K. and Diffley,J.F. (1996) An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature, 379, 180–182. [DOI] [PubMed] [Google Scholar]
- Coleman T.R., Carpenter,P.B. and Dunphy,W.G. (1996) The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell, 87, 53–63. [DOI] [PubMed] [Google Scholar]
- Delidakis C. and Kafatos,F.C. (1989) Amplification enhancers and replication origins in the autosomal chorion gene cluster of Drosophila. EMBO J., 8, 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J.F. (2001) DNA replication: building the perfect switch. Curr. Biol., 11, R367–R370. [DOI] [PubMed] [Google Scholar]
- Diffley J.F., Cocker,J.H., Dowell,S.J. and Rowley,A. (1994) Two steps in the assembly of complexes at yeast replication origins in vivo. Cell, 78, 303–316. [DOI] [PubMed] [Google Scholar]
- Donaldson A.D., Fangman,W.L. and Brewer,B.J. (1998) Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev., 12, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey D.D., Zhu,J., Carlson,D., Sharma,K. and Huberman,J.A. (1994) Three ARS elements contribute to the ura4 replication origin in the fission yeast, Schizosaccharomyces pombe. EMBO J., 13, 3638–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey D.D., Kim,S.M., Todorov,I.T. and Huberman,J.A. (1996) Large, complex modular structure of a fission yeast DNA replication origin. Curr. Biol., 6, 467–473. [DOI] [PubMed] [Google Scholar]
- Fantes P. (1979) Epistatic gene interactions in the control of division in fission yeast. Nature, 279, 428–430. [DOI] [PubMed] [Google Scholar]
- Gilbert D.M. (2001) Making sense of eukaryotic DNA replication origins. Science, 294, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M. and Antequera,F. (1999) Organization of DNA replication origins in the fission yeast genome. EMBO J., 18, 5683–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y., Toda,T. and Yanagida,M. (1984) The NDA3 gene of fission yeast encodes β-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell, 39, 349–358. [DOI] [PubMed] [Google Scholar]
- Hofmann J.F. and Beach,D. (1994) cdt1 is an essential target of the Cdc10/Sct1 transcription factor: requirement for DNA replication and inhibition of mitosis. EMBO J., 13, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M.T. and Stachow,C. (1990) Transformation of Schizosaccharo myces pombe by electroporation. Nucleic Acids Res., 18, 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y. (1997) A DNA helicase activity is associated with an MCM4, -6 and -7 protein complex. J. Biol. Chem., 272, 24508–24513. [DOI] [PubMed] [Google Scholar]
- Kelly T.J., Martin,G.S., Forsburg,S.L., Stephen,R.J., Russo,A. and Nurse,P. (1993) The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell, 74, 371–382. [DOI] [PubMed] [Google Scholar]
- Kim S.M. and Huberman,J.A. (1998) Multiple orientation-dependent, synergistically interacting, similar domains in the ribosomal DNA replication origin of the fission yeast, Schizosaccharomyces pombe. Mol. Cell. Biol., 18, 7294–7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.M. and Huberman,J.A. (1999) Influence of a replication enhancer on the hierarchy of origin efficiencies within a cluster of DNA replication origins. J. Mol. Biol., 288, 867–882. [DOI] [PubMed] [Google Scholar]
- Kong D. and DePamphilis,M.L. (2001) Site-specific DNA binding of the Schizosaccharomyces pombe origin recognition complex is determined by the Orc4 subunit. Mol. Cell. Biol., 21, 8095–8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D. and DePamphilis,M.L. (2002) Site-specific ORC binding, pre-replication complex assembly and DNA synthesis at Schizosaccharomyces pombe replication origins. EMBO J., 21, 5567–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenburger E.M., Keller,C. and Knippers,R. (2002) Identification of a binding region for human origin recognition complex proteins 1 and 2 that coincides with an origin of DNA replication. Mol. Cell. Biol., 22, 1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Moon,K.Y., Jiang,Y. and Hurwitz,J. (2001) The Schizosaccharomyces pombe origin recognition complex interacts with multiple AT-rich regions of the replication origin DNA by means of the AT-hook domains of the spOrc4 protein. Proc. Natl Acad. Sci. USA, 98, 13589–13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marahrens Y. and Stillman,B. (1992) A yeast chromosomal origin of DNA replication defined by multiple functional elements. Science, 255, 817–823. [DOI] [PubMed] [Google Scholar]
- Maundrell K., Hutchison,A. and Shall,S. (1988) Sequence analysis of ARS elements in fission yeast. EMBO J., 7, 2203–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Nguyen V.Q., Co,C. and Li,J.J. (2001) Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature, 411, 1068–1073. [DOI] [PubMed] [Google Scholar]
- Nishitani H., Lygerou,Z., Nishimoto,T. and Nurse,P. (2000) The Cdt1 protein is required to license DNA for replication in fission yeast. Nature, 404, 625–628. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux,P. and Nasmyth,K. (1976) Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet., 146, 167–178. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Takahashi,T. and Masukata,H. (1999) Association of fission yeast Orp1 and Mcm6 proteins with chromosomal replication origins. Mol. Cell. Biol., 19, 7228–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno Y., Okazaki,T. and Masukata,H. (1997) Identification of a predominant replication origin in fission yeast. Nucleic Acids Res., 25, 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno Y., Satoh,H., Sekiguchi,M. and Masukata,H. (1999) Clustered adenine/thymine stretches are essential for function of a fission yeast replication origin. Mol. Cell. Biol., 19, 6699–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J.A., Kim,S.M. and Huberman,J.A. (1998) Ribosomal DNA replication in the fission yeast, Schizosaccharomyces pombe. Exp. Cell Res., 238, 220–230. [DOI] [PubMed] [Google Scholar]
- Takahashi T. and Masukata,H. (2001) Interaction of fission yeast ORC with essential adenine/thymine stretches in replication origins. Genes Cells, 6, 837–849. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu,Z., Fu,X. and Liang,C. (2002) Noc3p, a bHLH protein, plays an integral role in the initiation of DNA replication in budding yeast. Cell, 109, 849–860. [DOI] [PubMed] [Google Scholar]
- Zhu J., Carlson,D.L., Dubey,D.D., Sharma,K. and Huberman,J.A. (1994) Comparison of the two major ARS elements of the ura4 replication origin region with other ARS elements in the fission yeast, Schizosaccharomyces pombe. Chromosoma, 103, 414–422. [DOI] [PubMed] [Google Scholar]
- Zou L. and Stillman,B. (2000) Assembly of a complex containing Cdc45p, replication protein A and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p–Dbf4p kinase. Mol. Cell. Biol., 20, 3086–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]