Abstract
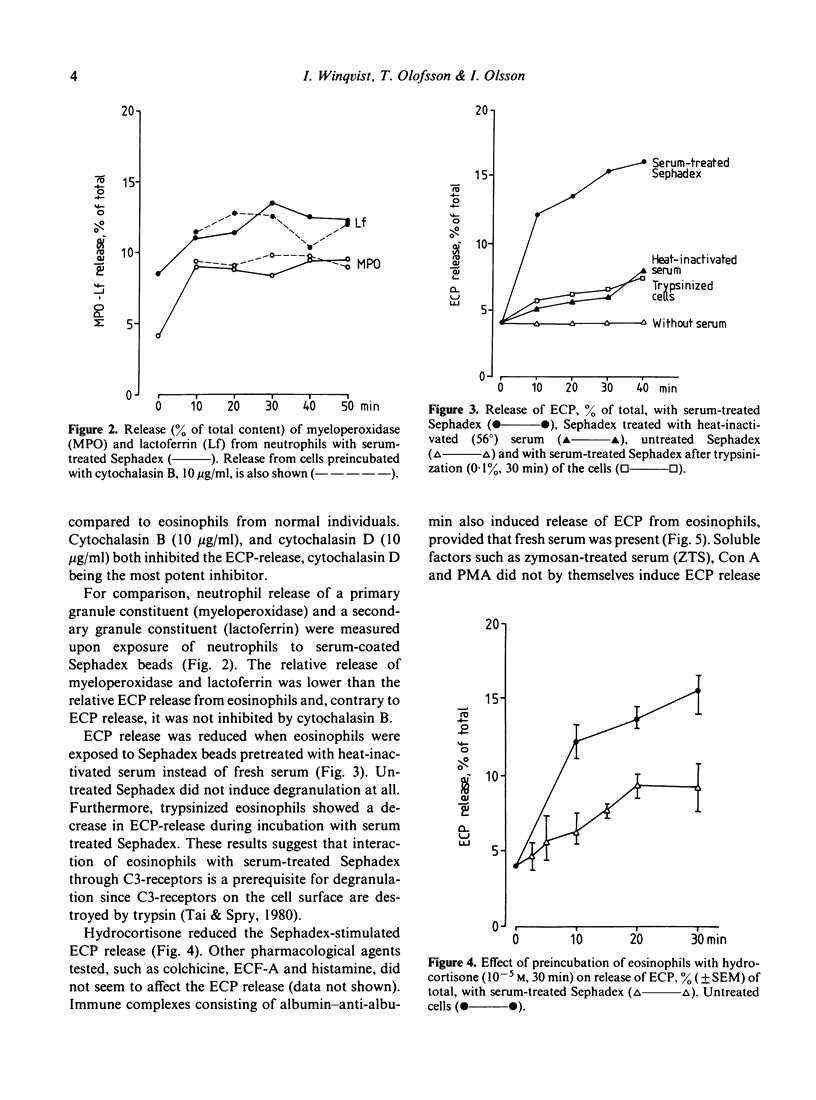
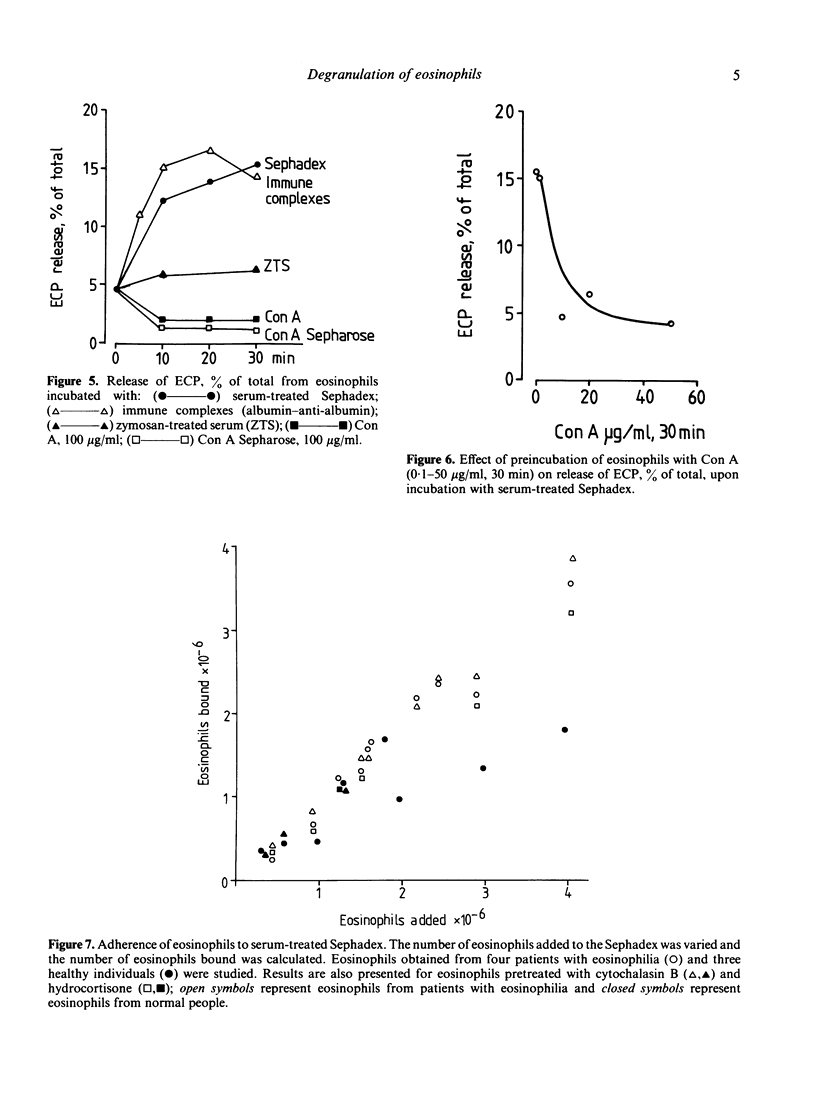
Mechanisms for degranulation in human eosinophils were evaluated. Release of eosinophil cationic protein (ECP), a unique eosinophil granule constituent, was measured upon exposure of purified eosinophils to a large surface consisting of Sephadex beads coated with serum, which leads to complement activation. Extracellular release of approximately 15% of the cellular ECP occurred both with eosinophils from patients with eosinophilia and normal people. Almost all eosinophils isolated from patients with eosinophilia and normal people adhered to serum-treated Sephadex. The data suggest that interaction through C3 receptors is a prerequisite for ECP release from eosinophils when exposed to serum-treated Sephadex. Both cytochalasin B, cytochalasin D and hydrocortisone reduced the release of ECP. Neither the cytochalasins nor hydrocortisone inhibited the adherence of eosinophils to the Sephadex beads. Thus the inhibitory effect of these agents on ECP release is a direct effect on the degranulation process. ECF-A, histamine and colchicine did not affect the release mechanism. No direct relationship was found between degranulation and oxidative burst inasmuch as some soluble mediators induced a high respiratory burst without a concomitant ECP release. Our data suggest that mechanisms for degranulation are not fully identical in eosinophils and neutrophils.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. C., Hill J. S., Hairfield W. M., Mullarkey M. F. Effects of corticosteroids on eosinophil chemotaxis and adherence. J Clin Invest. 1981 Jan;67(1):28–36. doi: 10.1172/JCI110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A. R., Kay B. Enhancement of human eosinophil complement receptors by pharmacologic mediators. J Immunol. 1978 Oct;121(4):1245–1250. [PubMed] [Google Scholar]
- Bass D. A., Grover W. H., Lewis J. C., Szejda P., DeChatelet L. R., McCall C. E. Comparison of human eosinophils from normals and patients with eosinophilia. J Clin Invest. 1980 Dec;66(6):1265–1273. doi: 10.1172/JCI109978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Wassom D. L., Gleich G. J., Loegering D. A., David J. R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979 Jan;122(1):221–229. [PubMed] [Google Scholar]
- Dahl R., Venge P., Olsson I. Variations of blood eosinophils and eosinophil cationic protein in serum in patients with bronchial asthma. Studies during inhalation challenge test. Allergy. 1978 Aug;33(4):211–215. doi: 10.1111/j.1398-9995.1978.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Fredens K., Dahl R., Venge P. The Gordon phenomenon induced by the eosinophil cationic protein and eosinophil protein X. J Allergy Clin Immunol. 1982 Nov;70(5):361–366. doi: 10.1016/0091-6749(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Gleich G. J. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980 Jan;42(1):35–43. [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. A., Kueppers F., Bajaj S. P., Mann K. G. Physiochemical and biological properties of the major basic protein from guinea pig eosinophil granules. J Exp Med. 1974 Aug 1;140(2):313–332. doi: 10.1084/jem.140.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. A., Maldonado J. E. Identification of a major basic protein in guinea pig eosinophil granules. J Exp Med. 1973 Jun 1;137(6):1459–1471. doi: 10.1084/jem.137.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. A., Mann K. G., Maldonado J. E. Comparative properties of the Charcot-Leyden crystal protein and the major basic protein from human eosinophils. J Clin Invest. 1976 Mar;57(3):633–640. doi: 10.1172/JCI108319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner I. Separation of human eosinophils in density gradients of polyvinylpyrrolidone-coated silica gel (Percoll). Immunology. 1980 May;40(1):133–136. [PMC free article] [PubMed] [Google Scholar]
- Kay A. B. The role of the eosinophil. J Allergy Clin Immunol. 1979 Aug;64(2):90–104. doi: 10.1016/0091-6749(79)90042-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McLaren D. J., McKean J. R., Olsson I., Venges P., Kay A. B. Morphological studies on the killing of schistosomula of Schistosoma mansoni by human eosinophil and neutrophil cationic proteins in vitro. Parasite Immunol. 1981 Winter;3(4):359–373. doi: 10.1111/j.1365-3024.1981.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe D. D., Gadek J. E., Raphael G. D., Frank M. M., Kaplan A. P., Kaliner M. Human eosinophil adherence to serum-treated sepharose: granule-associated enzyme release and requirement for activation of the alternative complement pathway. J Immunol. 1977 Nov;119(5):1744–1750. [PubMed] [Google Scholar]
- Miranda A. F., Godman G. C., Deitch A. D., Tanenbaum S. W. Action of cytochalasin D on cells of established lines. I. Early events. J Cell Biol. 1974 May;61(2):481–500. doi: 10.1083/jcb.61.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. F., Godman G. C., Tanenbaum S. W. Action of cytochalasin D on cells of established lines. II. Cortex and microfilaments. J Cell Biol. 1974 Aug;62(2):406–423. doi: 10.1083/jcb.62.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Ohlsson K., Gustavsson A. Serum and plasma myeloperoxidase, elastase and lactoferrin content in acute myeloid leukaemia. Scand J Haematol. 1979 May;22(5):397–406. doi: 10.1111/j.1600-0609.1979.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Venge P., Spitznagel J. K., Lehrer R. I. Arginine-rich cationic proteins of human eosinophil granules: comparison of the constituents of eosinophilic and neutrophilic leukocytes. Lab Invest. 1977 May;36(5):493–500. [PubMed] [Google Scholar]
- Palek J., Liu S. C. Dependence of spectrin organization in red blood cell membranes on cell metabolism: implications for control of red cell shape, deformability, and surface area. Semin Hematol. 1979 Jan;16(1):75–93. [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. Enzymes altering the binding capacity of human blood eosinophils for IgG antibody-coated erythrocytes (EA). Clin Exp Immunol. 1980 Apr;40(1):206–219. [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. The mechanisms which produce vacuolated and degranulated eosinophils. Br J Haematol. 1981 Oct;49(2):219–226. doi: 10.1111/j.1365-2141.1981.tb07218.x. [DOI] [PubMed] [Google Scholar]
- Venge P., Roxin L. E., Olsson I. Radioimmunoassay of human eosinophil cationic protein. Br J Haematol. 1977 Nov;37(3):331–335. doi: 10.1111/j.1365-2141.1977.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Wasserman S. I., Goetzl E. J., Austen K. F. Inactivation of slow reacting substance of anaphylaxis by human eosinophil arylsulfatase. J Immunol. 1975 Feb;114(2 Pt 1):645–649. [PubMed] [Google Scholar]
- Winqvist I., Olofsson T., Olsson I., Persson A. M., Hallberg T. Altered density, metabolism and surface receptors of eosinophils in eosinophilia. Immunology. 1982 Nov;47(3):531–539. [PMC free article] [PubMed] [Google Scholar]
- Winqvist I., Olsson I., Werner S., Stenstam M. Variations of cationic proteins from eosinophil leukocytes in food intolerance and allergic rhinitis. Allergy. 1981 Aug;36(6):419–423. doi: 10.1111/j.1398-9995.1981.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Zeiger R. S., Colten H. R. Histaminase release from human eosinophils. J Immunol. 1977 Feb;118(2):540–543. [PubMed] [Google Scholar]


