Abstract
Infections with parasitic helminths are important causes of morbidity and mortality worldwide. New drugs that are parasite specific and minimally toxic to the host are needed to counter these infections effectively. Here we report the finding of a previously unidentified compound, nafuredin, from Aspergillus niger. Nafuredin inhibits NADH-fumarate reductase (complexes I + II) activity, a unique anaerobic electron transport system in helminth mitochondria, at nM order. It competes for the quinone-binding site in complex I and shows high selective toxicity to the helminth enzyme. Moreover, nafuredin exerts anthelmintic activity against Haemonchus contortus in in vivo trials with sheep. Thus, our study indicates that mitochondrial complex I is a promising target for chemotherapy, and nafuredin is a potential lead compound as an anthelmintic isolated from microorganisms.
Helminthiasis is a crucial problem worldwide, because it is not only a major cause of human morbidity in the tropics as well as temperate climates (1), but is responsible also for enormous economic losses in livestock animals (2). In addition, recent results suggest that helminth infections impair the immune response of the host to HIV and tuberculosis and might contribute to the spread of these diseases (3). Furthermore, the emergence of resistance against generally used anthelmintics makes the problem more serious (2, 4). Therefore, the development of novel and potent anthelmintics is urgent. Different classes of broad-spectrum agents such as levamisole, pyrantel, benzimidazoles, and the macrocyclic lactones (e.g., avermectin) have been used widely to treat helminthiasis (5). However, the discovery of potent new classes of anthelmintics has been rare after our discovery of avermectin (6), which was isolated from Streptomyces avermitilis and has been used to treat filariasis worldwide. Regular treatment of livestock causes the emergence of resistance against these classes of drugs. Therefore, there is a steady and urgent need to develop novel anthelmintics and to find new potential targets for such compounds.
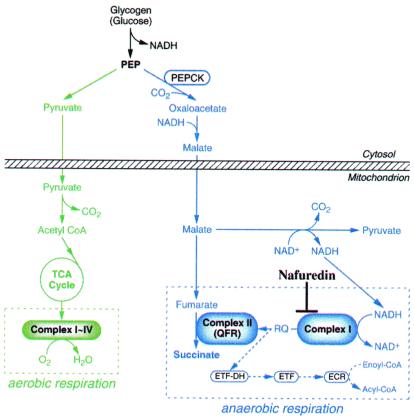
Because helminths have exploited a variety of energy-transducing systems in their adaptation to the peculiar habitats in their hosts, differences in energy metabolisms between the host and helminths are attractive therapeutic targets of helminthiasis. NADH-fumarate reductase is part of a unique respiratory system in parasitic helminths (7–10) and is the terminal step of the phosphoenolpyruvate carboxykinase-succinate pathway, which is found in many anaerobic organisms (11–13). The composition and linear sequential order of the respiratory components of NADH-fumarate reductase have been elucidated with mitochondria from the parasitic nematode, Ascaris suum (Fig. 1; refs. 14–17). Electrons from NADH are accepted by rhodoquinone through complex I (NADH-rhodoquinone oxidoreductase), and then transferred to fumarate through complex II (rhodoquinol-fumarate reductase). This anaerobic electron transport couples site I phosphorylation in complex I by translocating protons across the inner mitochondrial membrane, providing ATP even in the absence of oxygen. Although this system has been thought to be a good target for developing anthelmintics, only a few compounds have been reported [e.g., bithionol (18) and thiabendazole (19)] to be inhibitors of rhodoquinol-fumarate reductase, and the relationship between their enzyme inhibitions and anthelmintic activities is not clear because their inhibitory activities are weak.
Figure 1.
NADH-fumarate reductase system in A. suum adult
mitochondria and inhibition site of nafuredin. In the
phosphoenolpyruvate carboxykinase (PEPCK)-succinate
pathway, phosphoenolpyruvate (PEP) produced by a
glycolytic process is carboxylated to form oxaloacetate and is then
reduced to malate. The cytosolic malate is transported into the
mitochondria, where it is first reduced to fumarate, and finally to
succinate by the rhodoquinol-fumarate reductase activity of complex II.
The terminal step is catalyzed by the NADH-fumarate reductase system
comprising complex I, rhodoquinone (RQ), and complex II, as described
in the text. Aerobic and anaerobic respiratory chains are boxed in
broken lines. Electron-transfer flavoprotein (ETF)
dehydrogenase:rhodoquinone branch
( ) is also included. ETF-DH,
ETF-dehydrogenase; ECR, enoyl-CoA reductase.
) is also included. ETF-DH,
ETF-dehydrogenase; ECR, enoyl-CoA reductase.
Therefore, to find new-class anthelmintics, we carried out screening for inhibitors of NADH-fumarate reductase by using A. suum mitochondria.
Materials and Methods
Fermentation and Purification of Nafuredin.
Aspergillus niger FT-0554 was cultured in 500-ml Erlenmeyer flasks containing 100 ml of a production medium [2.4% potato dextrose broth (Difco) and 25% natural seawater (34%0 salinity)] on a rotary shaker (210 rpm) at 27°C for 6 days. The mycelium collected by filtering the cultured broth was extracted first with acetone and then with n-hexane. The n-hexane layer was evaporated to dryness and the crude extract was chromatographed on a silica gel column eluted with n-hexane and ethyl acetate (stepwise elution). The active fraction eluted with n-hexane-ethyl acetate (10:2, vol/vol) was evaporated; finally, nafuredin was obtained as a white powder by precipitation in n-hexane. Nafuredin: [α]D27 + 89.9 (c0.1, chloroform); UV λmax (ethanol) 253 nm (ɛ 23,000); IR νmax (KBr) 3,440, 1,740 cm−1; MS (high-resolution fast atom bombardment) calculated for C22H33O4 [M + H]+ 361.2379, found 361.2374.
Enzyme Assays.
The rhodoquinol-fumarate reductase activity was measured in assay mixtures containing 50 mM potassium phosphate (pH 7.4), 0.05% (wt/vol) sucrose monolaurate, 10 mM β-d-glucose, 20 units of glucose oxidase, 26 units of catalase, 100 μM decyl-rhodoquinone, and 1 mM sodium borohydride. The reaction is started by the addition of sodium fumarate (5 mM). The millimolar extinction coefficient of rhodoquinone is 7.9 at 283 nm. Synthetic procedures for decyl-rhodoquinone will be described elsewhere. The NADH-fumarate reductase activity was measured in assay mixtures containing 50 mM potassium phosphate (pH 7.2), 10 mM β-d-glucose, 20 units of glucose oxidase, 26 units of catalase, and 200 μM NADH. The reaction is started by the addition of sodium fumarate (5 mM). The absorbance change at 340 nm (millimolar extinction coefficient of 6.2 for NADH) was followed. Other enzyme activities were measured as described (16).
Sheep Studies.
Methods for evaluating anthelmintic activity against Haemonchus contortus in sheep were performed as described (20). Sheep were infected orally with 5,000 L3 larvae of H. contortus. Three sheep were treated orally with 2 mg/kg body weight of nafuredin each dissolved at 1% in an emulsifying solution (1 ml Cremophor EL/glycerinformal 1: 2, 7 ml H2O), and two sheep were treated only with the emulsifying solution as controls. Fecal egg counts were monitored until 11 days after treatment in a way similar to that described (20).
Results and Discussion
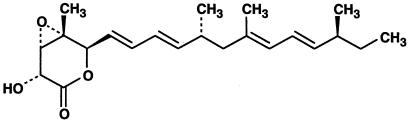
We have screened more than 10,000 culture broths of fungi and actinomycetes that showed inhibition against NADH-fumarate reductase of A. suum and found an inhibitor, nafuredin, from the culture broth of A. niger FT-0554 isolated from a marine sponge. Nafuredin has a structure of epoxy-δ-lactone with a methylated olefinic side chain (Fig. 2). Details of the finding and structure elucidation of nafuredin will be presented elsewhere.
Figure 2.
Structure of nafuredin with absolute configuration.
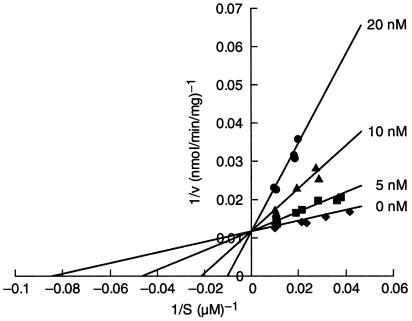
To study the mode of action of nafuredin, we examined its effect on various electron-transport activities of the A. suum NADH-fumarate reductase system (Table 1). Nafuredin inhibited NADH-fumarate reductase and NADH-rhodoquinone oxidoreductase activities with IC50 values of 12 nM and 24 nM, respectively, but it showed only weak inhibition of rhodoquinol-fumarate reductase (IC50 = 80 μM). In these assays, we used synthetic short-chain rhodoquinone (or rhodoquinol) possessing an n-decyl group as an alkyl side chain. The kinetic analysis revealed that the inhibition is uncompetitive with NADH (data not shown) and competitive with rhodoquinone (Fig. 3), indicating that the site of inhibition of nafuredin is the quinone-binding domain in complex I. Furthermore, nafuredin inhibits complex I (NADH-ubiquinone oxidoreductase) in L2 larvae of A. suum, which possess aerobic energy metabolism as in mammals (16, 21), at the same concentration range (IC50 = 8.9 nM), suggesting that nafuredin is effective against both adult and larval stages. In contrast, the IC50 value for rat liver complex I was more than 1,000 times higher than for the A. suum complex I (Table 1). Thus, nafuredin is a markedly selective inhibitor of helminth complex I and expected to be an excellent anthelmintic.
Table 1.
Effects of nafuredin on electron transport enzymes
| IC50 (nM)
|
|||||
|---|---|---|---|---|---|
| Complex | Ascaris suum (adult) | Ascaris suum (L2) | Haemonchus contortus (adult) | Rat liver | |
| NADH-fumarate reductase | I + II | 12 | — | — | 1,000 |
| NADH-ubiquinone reductase | I | 8 | 8.9 | 86 | 10,000 |
| NADH-rhodoquinone reductase | I | 24 | 9.0 | 195 | >100,000 |
| Rhodoquinol-fumarate reductase | II | 80,000 | — | — | — |
| Succinate-ubiquinone reductase | II | >100,000 | — | — | >100,000 |
—: Not tested.
Figure 3.
Kinetic analysis of nafuredin inhibition of complex I for rhodoquinone. The catalytic activities were expressed as nmol of NADH oxidized per min per mg of protein. The assays were performed in 50 mM potassium phosphate buffer (pH 7.2), 1 mM KCN, 200 μM NADH, and 30 μg A. suum mitochondria, at 30°C. The enzymatic reaction was started by the addition of decyl-rhodoquinone. The absorbance change at 340 nm (millimolar extinction coefficient of 6.2 for NADH) was followed.
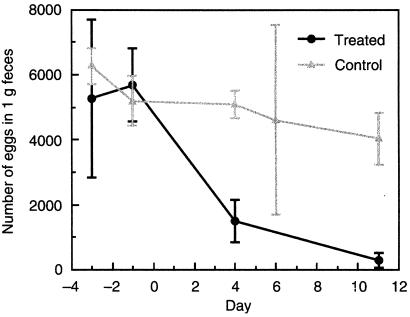
On the basis of these observations, we next analyzed the efficacy of nafuredin by using an animal model. In sheep studies, 2 mg/kg [given orally (p.o.)] of nafuredin exerted anthelmintic activity against H. contortus (wireworm) by suppressing the egg output of female worms within 11 days after treatment (Fig. 4). A greater than 90% egg reduction was observed at day 11. Furthermore, egg output was completely suppressed when the sheep were treated similarly again 1 week after the first treatment (data not shown). This anthelmintic activity may be caused by the hampered energy metabolism of the parasite, because complex I from H. contortus was also sensitive to nafuredin, although the inhibitions were relatively weaker than those for A. suum (Table 1). Moreover, nafuredin was effective in mice infected with dwarf tapeworm, Hymenolepis nana (data not shown). There were no signs of any side effects and no loss of body weight during the whole tests in either sheep (2 mg/kg p.o.) or mice (50 mg/kg p.o. and i.p.).
Figure 4.
Results of H. contortus infectious experiments in sheep. Eggs per gram of feces from H. contortus infected sheep on different days before and after oral treatment with 2 mg/kg nafuredin compared with untreated infected sheep are shown. Values are the means of two experiments (controls) or three experiments (treated animals) ± SD.
Although nafuredin has the common structural features of ordinary complex I inhibitor, that is, a polar heterocyclic ring and hydrophobic tail moiety (22), this compound shows markedly selective inhibition of helminth complex I. In mammals, complex I comprises 43 different subunits with a molecular mass of about 1,000 kDa. Despite recent structural studies, little is known about the electron-transport pathway, proton-translocation mechanism, and mode of action of a large number of specific inhibitors that compete for the quinone-binding site. Kinetic studies suggest that these inhibitors can be grouped into three classes and that hydrophobic inhibitors share a common large binding domain with partially overlapping sites (22–24). Participation of PSST subunit to this inhibitor-binding site in mammalian complex I has been demonstrated (25). Helminth complex I may contain this inhibitor-binding domain because the NADH-cytochrome c oxidoreductase activity of A. suum mitochondria is sensitive to ordinary complex I inhibitors such as rotenone and piericidin A (26). However, specific inhibition by nafuredin indicates that the structure of the domain in helminth complex I differs somewhat from that of its mammalian counterparts. The present study clearly shows that complex I is a promising target for anthelmintics. Studies on the mode of action of nafuredin will provide new insights into the structure-function relationship of helminth complex I.
Acknowledgments
This work was supported partly by a Grant-in-Aid for Encouragement of Young Scientists from Japan Society for the Promotion of Science (12771373 to H.U.), and by a Grant-in-Aid from the Ministry of Education, Science, Culture and Sports, Japan (11470065 to K.K.). The financial support by Japan Keirin Association and by a Grant-in-Aid for research on emerging and re-emerging infectious diseases from the Ministry of Health and Welfare are also gratefully acknowledged.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011524698.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011524698
References
- 1.Bundy D A P, Silva N R. Br Med Bull. 1998;54:421–432. doi: 10.1093/oxfordjournals.bmb.a011698. [DOI] [PubMed] [Google Scholar]
- 2.Waller P J. Vet Parasitol. 1997;72:391–412. doi: 10.1016/s0304-4017(97)00107-6. [DOI] [PubMed] [Google Scholar]
- 3.Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N, Beyers A D. Immunol Today. 1999;20:485–487. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 4.Sangster N C, Gill J. Parasitol Today. 1999;15:141–146. doi: 10.1016/s0169-4758(99)01413-1. [DOI] [PubMed] [Google Scholar]
- 5.Martin R J, Robertson A P, Bjorn H. Parasitology. 1997;114:S111–S124. [PubMed] [Google Scholar]
- 6.Burg R W, Miller B M, Baker E E, Birnbaum J, Currie S A, Hartman R, Kong Y L, Monaghan R L, Olson G, Putter I, et al. Antimicrob Agents Chemother. 1979;15:361–367. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boveris A, Hertig C M, Turrens J F. Mol Biochem Parasitol. 1986;19:163–169. doi: 10.1016/0166-6851(86)90121-0. [DOI] [PubMed] [Google Scholar]
- 8.Takamiya S, Wang H, Hiraishi A, Yu Y, Hamajima F, Aoki T. Arch Biochem Biophys. 1994;312:142–150. doi: 10.1006/abbi.1994.1292. [DOI] [PubMed] [Google Scholar]
- 9.Van Hellemond J J, Tielens A G M. Biochem J. 1994;304:321–331. doi: 10.1042/bj3040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fioravanti C F, Walker D J, Sandhu P S. Parasitol Res. 1998;84:777–782. doi: 10.1007/s004360050487. [DOI] [PubMed] [Google Scholar]
- 11.Saz H J. Ann Rev Physiol. 1981;43:323–341. doi: 10.1146/annurev.ph.43.030181.001543. [DOI] [PubMed] [Google Scholar]
- 12.Oya H, Kita K. In: Comparative Biochemistry of Parasitic Helminths. Bennet E-M, Behm C, Bryant C, editors. London: Chapman & Hall; 1988. pp. 35–53. [Google Scholar]
- 13.Komuniecki R, Harris B G. In: Biochemistry and Molecular Biology of Parasites. Marr J, Mueller M, editors. London: Academic; 1995. pp. 49–66. [Google Scholar]
- 14.Kita K, Hirawake H, Takamiya S. Int J Parasitol. 1997;27:617–630. doi: 10.1016/s0020-7519(97)00016-7. [DOI] [PubMed] [Google Scholar]
- 15.Kuramochi T, Hirawake H, Kojima S, Takamiya S, Furushima R, Aoki T, Komuniecki R, Kita K. Mol Biochem Parasitol. 1994;68:177–187. doi: 10.1016/0166-6851(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 16.Saruta F, Kuramochi T, Nakamura K, Takamiya S, Yu Y, Aoki T, Sekimizu K, Kojima S, Kita K. J Biol Chem. 1995;270:928–932. doi: 10.1074/jbc.270.2.928. [DOI] [PubMed] [Google Scholar]
- 17.Amino H, Wang H, Hirawake H, Saruta F, Mizuchi D, Mineki R, Shindo N, Murayama K, Takamiya S, Aoki T, et al. Mol Biochem Parasitol. 2000;106:63–76. doi: 10.1016/s0166-6851(99)00200-5. [DOI] [PubMed] [Google Scholar]
- 18.Ikuma K, Makimura M, Murakoshi Y. Yakugaku Zasshi. 1993;113:663–669. doi: 10.1248/yakushi1947.113.9_663. [DOI] [PubMed] [Google Scholar]
- 19.Köhler P, Bachmann R. Mol Pharmacol. 1978;14:155–163. [PubMed] [Google Scholar]
- 20.Von Samson-Himmlestjerna G, Harder A, Schnieder T, Kalbe J, Mencke N. Parasitol Res. 2000;86:194–199. doi: 10.1007/s004360050031. [DOI] [PubMed] [Google Scholar]
- 21.Takamiya S, Kita K, Wang H, Weinstein P P, Hiraishi A, Oya H, Aoki T. Biochim Biophys Acta. 1993;1141:65–74. doi: 10.1016/0005-2728(93)90190-q. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi H. Biochim Biophys Acta. 1998;1364:236–244. doi: 10.1016/s0005-2728(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 23.Degli Espsti M. Biochim Biophys Acta. 1998;1364:222–235. doi: 10.1016/s0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 24.Okun J G, Lummen P, Brandt U. J Biol Chem. 1999;274:2625–2630. doi: 10.1074/jbc.274.5.2625. [DOI] [PubMed] [Google Scholar]
- 25.Schuler F, Yano T, Bernardo S, Yagi T, Yankovskaya V, Singer T, Casida J E. Proc Natl Acad Sci USA. 1999;96:4149–4153. doi: 10.1073/pnas.96.7.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takamiya S, Furushima R, Oya H. Mol Biochem Parasitol. 1984;13:121–134. doi: 10.1016/0166-6851(84)90107-5. [DOI] [PubMed] [Google Scholar]