Abstract
The Tat pathway is a major route for protein export in prokaryotes and for protein targeting to thylakoids in chloroplasts. Based on in vitro studies, protein translocation through this pathway is thought to be strictly dependent on a transmembrane ΔpH. In this paper, we assess the ΔpH sensitivity of the Tat pathway in vivo. Using Chlamydomonas reinhardtii, we observed changes in the efficiency of thylakoid targeting in vivo by mutating the Tat signal of the Rieske protein. We then employed two endogenous pH probes located on the lumen side of the thylakoid membranes to estimate spectroscopically the ΔpH in vivo. Using experimental conditions in which the trans-thylakoid ΔpH was almost zero, we found no evidence for a ΔpH dependence of the Tat pathway in vivo. We confirmed this observation in higher plants using attached barley leaves. We conclude that the Tat pathway does not require a ΔpH under physiological conditions, but becomes ΔpH sensitive when probed in vitro/in organello because of the loss of some critical intracellular factors.
Keywords: chloroplast/ΔpH/protein import/Rieske protein/Tat pathway
Introduction
Protein translocation across lipid bilayers plays a fundamental role in cellular metabolism as it allows the import of newly synthesized secretory proteins in the endoplasmic reticulum (ER), the export of proteins in the bacterial periplasmic space and the import of nuclear-encoded proteins in mitochondria and chloroplasts. In the case of organelles, nuclear-encoded respiratory or photosynthetic proteins have to cross up to three membranes in order to reach their final destination (reviewed in Wollman et al., 1999; Pfanner and Geisler, 2001). In plastids, which are separated from the cytosol by an outer and an inner envelope membrane, translocon complexes within the outer (TOC) and inner (TIC) membranes import precursor proteins into the stroma (reviewed in Reumann and Keegstra, 1999). Stromal proteins may then be targeted to thylakoid membranes through three distinct pathways that have bacterial homologues: the Sec, SRP and Tat pathways. A fourth pathway, considered as ‘spontaneous’, may be organelle specific (Michl et al., 1994). This diversity in thylakoid targeting pathways, each using a specific set of substrate proteins, was first demonstrated in vitro by competition experiments (e.g. Cline et al., 1993) and then confirmed in vivo with the isolation of plant mutants that were selectively impaired in the translocation of the SRP, Sec or Tat substrates (Voelker and Barkan, 1995; Settles et al., 1997; Amin et al., 1999; Motohashi et al., 2001). While the Sec and SRP pathways translocate polypeptides in their unfolded state, and therefore require the activity of soluble chaperones (reviewed in Mori and Cline, 2001; Robinson and Bolhuis, 2001), the Tat pathway has the unique ability to transport proteins in their folded state (Clark and Theg, 1997; Hynds et al., 1998; Santini et al., 2001; Thomas et al., 2001). Targeting of the Tat substrate proteins is specified by their N-terminus presequence, which is characterized by the presence of a twin arginine (RR) motif situated upstream of a hydrophobic stretch (Chaddock et al., 1995). The energetics of protein translocation also differ among the three pathways of bacterial origin. The Sec and SRP pathways require hydrolysis of nucleotide triphosphates, ATP in the case of Sec and GTP in the case of SRP (reviewed in Kouranov and Schnell, 1996), although a proton motive force may also play a role (Ernst et al., 1994; Yuan and Cline, 1994; Mant et al., 1995). In contrast, protein translocation via the Tat pathway only requires a ΔpH (Mould and Robinson, 1991; Cline et al., 1992).
Unlike substrate specificity, which has been assessed both in vitro and in vivo, the energetics of protein translocation by the various pathways have been assessed only in vitro using intact thylakoids or in organello. In this paper we address the question of the ΔpH dependence of the Tat pathway in vivo using the unicellular green alga Chlamydomonas reinhardtii, which is best suited for molecular genetics and biochemical approaches, and offers the same experimental opportunity as Chlorella sorokiniana (Finazzi and Rappaport, 1998; Rappaport et al., 1999) for the study and control of the trans-thylakoid ΔpH in vivo. In particular, the well developed genetics of C.reinhardtii have produced a number of mutants lacking the chloroplast ATPsynthase (Lemaire et al., 1986), which should have distinct ΔpH properties when compared with the wild type. Here, we focused on three major Tat passenger proteins: the 16 and 23 kDa subunits of the oxygen-evolving complex of photosystem II (PSII) (Mould and Robinson, 1991; Cline et al., 1992, 1993), and the Rieske subunit of the cytochrome b6f (cyt b6f) complex (Madueño et al., 1994; Molik et al., 2001). We demonstrate that targeting of any of these three proteins to the thylakoid membranes is ΔpH insensitive in vivo, a conclusion that challenges the conclusions on the energetic requirements of the Tat pathway previously proposed on the basis of in vitro studies.
Results
The Tat signal is important for thylakoid targeting of the Rieske protein in intact cells of C.reinhardtii
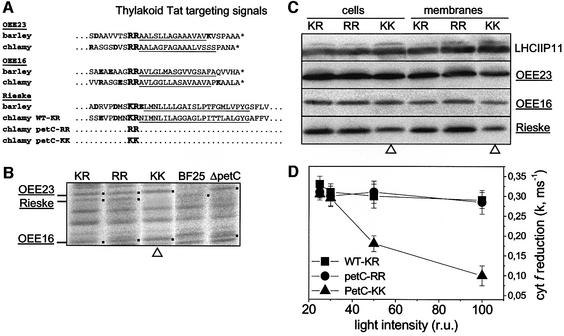
Figure 1A shows the barley and Chlamydomonas targeting sequences of the three Tat passenger proteins used in the present study. In contrast to its bacterial counterpart, which displays a regular (RR) Tat motif, the chloroplast Rieske protein exhibits an unusual KR signal. Nevertheless it is a bona fide Tat passenger protein, as demonstrated in vitro through competition experiments using vascular plant chloroplasts (Madueño et al., 1994; Molik et al., 2001). In order to assess the importance of this specific KR signature in the biogenesis and chloroplast targeting of the Rieske protein, we converted it to a canonical Tat motif or to a non-Tat motif (petC-RR and petC-KK, respectively, in Figure 1A).
Fig. 1. Consequences of Tat mutagenesis on chloroplast protein import in C.reinhardtii. (A) Tat motif of the OEE23, OEE16 and Rieske proteins (bold). The hydrophobic N-terminal α-helix is underlined and the cleavage site of the stroma peptidase is indicated by an asterisk. (B) Autoradiograph of membrane-associated protein synthesized during a 10 min in vivo pulse [14C]acetate labelling in the presence of 150 µg/ml chloramphenicol (inhibitor of chloroplast-encoded protein synthesis). KR, WT-KR wild-type strain with Rieske KR motif; RR, petC-RR Rieske mutant with RR motif; KK, petC-KK Rieske mutant with KK motif; BF25, mutant lacking membrane-associated OEE23 and OEE16; ΔpetC, petC-Δ1 Rieske deletion mutant used as the recipient strain for mutagenesis. The dots indicate predicted protein positions. Tat substrates are underlined. (C) Immunodetection of protein accumulation in whole cells (cells) and thylakoid membranes (membranes). (D) Light intensity dependence of the cyt f reduction rate. A decrease in the rate of electron transfer at saturating light intensity suggests the occurrence of multiple turnovers in b6f complexes and therefore a reduction in the accumulation of functional complexes.
The efficiency of translocation of the Rieske protein by the Tat pathway was probed in pulse-labelling experiments in which we labelled whole cells of C.reinhardtii for 10 min with [14C]acetate, and then purified their thylakoid membranes and analysed the membrane protein content by SDS–PAGE and autoradiography. The labelled bands of the autoradiogram corresponded to those proteins that were translated and successfully targeted to the thylakoid membranes during the time of the pulse (Figure 1B). The three Tat passenger proteins OEE23, OEE16 and Rieske are readily identified on the autoradiogram shown in Figure 1B by their absence from the membranes of the BF25 and petC-Δ1 mutants: OEE23 and OEE16 are absent in the BF25 mutant (de Vitry et al., 1989) and the Rieske protein is absent in the petC-Δ1 mutant (de Vitry et al., 1999). We observed that the rate of labelling of the Rieske protein was similar in the RR and KR lanes, which means that replacement of the KR targeting signal by an RR motif did not modify the efficiency of membrane targeting of the Rieske protein. In marked contrast, the labelled band corresponding to the Rieske protein remained below detection in the KK lane, which indicated that a KK substitution caused a drastic fall in the efficiency of membrane targeting of the Rieske protein in vivo. We used specific antibodies to assess the steady-state level of accumulation of the Rieske protein in the thylakoid membranes of the three strains (Figure 1C). As expected from the fall in thylakoid targeting efficiency, we observed a lower accumulation of the mature Rieske protein in the KK strain than in the KR (wild type) and RR strains. This was observed in whole-cell extracts (ratio 0.39 ± 0.11, mean value of five experiments) as well as in thylakoid membrane fractions (ratio 0.31 ± 0.06, mean value of four experiments) with no significant accumulation of untranslocated Rieske protein in the chloroplast stroma (not shown). Translocation and accumulation of the other Tat substrates (OEE23 and OEE16) were similar in the three strains.
The reduced accumulation of the Rieske protein had consequences for cyt b6f activity. Consistent with PSI being in excess relative to the b6f complex in this mutant strain, the rate of cyt f reduction measured after a single turnover flash decreased as the light intensity increased. This is due to the increased occurrence of multiple turnovers in the cyt b6f in order to re-reduce the increased fraction of oxidized plastocyanin generated by PSI at higher light intensity. The rate of cyt f reduction started to decrease above a light intensity equal to 30% of the saturating value (Figure 1D), suggesting that the 1:1 PSI–b6f stoichiometry was attained when one-third of PSI was excited. This is in good agreement with the finding that ∼30% of the Rieske protein is accumulated in the mutant (Figure 1C).
Measurement of trans-thylakoid ΔpH in intact cells of C.reinhardtii
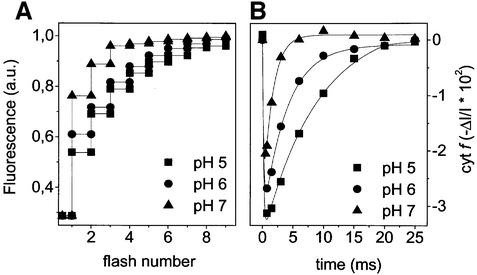
Having ascertained that the targeting specificity of the Tat pathway in C.reinhardtii chloroplasts is similar to what has previously been described in higher plant chloroplasts and bacteria, we undertook a characterization of its energetic requirements in vivo. To do this, we took advantage of the fact that a Δµ̃H+ is established across thylakoid membranes in dark-adapted green algae (Bennoun, 1982). Its composition and amplitude can be conveniently studied by measuring the response of two endogenous lumen-exposed pH probes that can be easily monitored spectroscopically in dark-adapted algae. Using this approach, the properties of Δµ̃H+ have been extensively characterized in C.sorokiniana (Finazzi and Rappaport, 1998; Rappaport et al., 1999), leading to the conclusion that ΔpH was the predominant component of Δµ̃H+ owing to ATP hydrolysis by the membrane-bound CF0–CF1 protein complex. It is known that a Δµ̃H+ is also established in dark-adapted cells of C.reinhardtii (Finazzi et al., 1997), but its detailed characterization had not yet been carried out. This characterization is presented in Figures 2 and 3. First, we measured the pH dependence of PSII- and cyt b6f-related activities in wild-type cells (Figure 2). Upon illumination by a short flash, charge separation occurs in PSII photochemical centres between a donor (P680) and an acceptor (Qa) molecule. This yields a state P680+ Qa–, which may or may not recombine to its original state: the faster that P680+ is reduced by a secondary donor (Tyr YD1161–), the less charge recombination takes place within the reaction centre and the longer lived is the reduced form of the primary acceptor Qa–. Since chlorophyll fluorescence increases when Qa is in a reduced state (Duysens and Sweers, 1963), the efficiency of charge separation after single turnover flashes can easily be monitored by fluorescence measurements. Figure 2A shows such an experiment where the fluorescence changes were recorded during a series of single turnover saturating flashes in the presence of the artificial tertiary donor hydroxylamine (HA), which destroys the manganese cluster, and 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU), an inhibitor of Qa– re-oxidation by the secondary acceptor Qb (Bennoun, 1970). These conditions slow down the re-reduction step of P680+, thus enhancing its pH dependence (Lavergne and Rappaport, 1998). The series of flash-induced fluorescence increases are presented at three different luminal pH values (set with permeant buffers as described in Materials and methods). We observed that the efficiency of charge separation decreased with the acidification of the luminal pH. This can be quantified by calculation of the fraction R of variable fluorescence increase on the first flash with respect to the maximum variable fluorescence increase reached at the end of the series of flashes. This fraction is much lower at pH 5 than at pH 7, as previously reported (Rappaport et al., 1999).
Fig. 2. Changes in PSII and cyt b6f activity as a function of pH in wild-type cells of C.reinhardtii. (A) Changes in the PSII-modulated chlorophyll fluorescence yield induced by a series of saturating flashes in the presence of DCMU and HA as a function of pH. The time between flashes was 200 ms, and fluorescence was sampled 50 and 100 ms after each actinic flash. (B) The kinetics of cyt f redox changes as a function of pH. Algae were excited with non-saturating flashes (20% of maximum intensity) in order to avoid multiple turnovers of the b6f complex and the generation of a substantial light-induced ΔpH. pH equilibration was performed as described in Materials and methods.
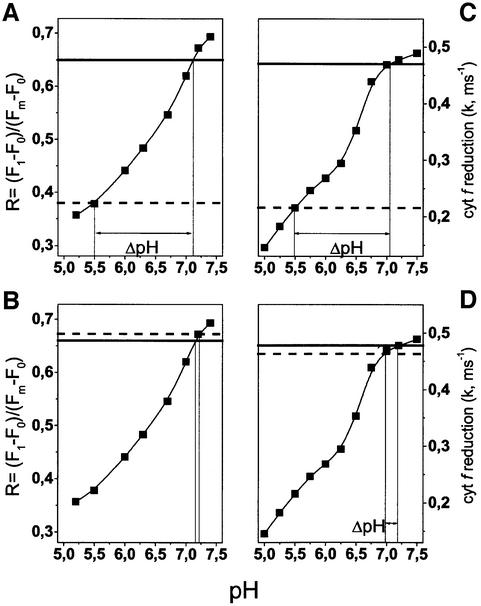
Fig. 3. Absolute pH dependence of PSII and cyt b6f activity in whole cells of C.reinhardtii. The pH dependence of the PSII-modulated chlorophyll fluorescence yield (A and B) and the rate of cyt f reduction (C and D) were calculated from traces as in Figure 2. The fluorescence yield is indicated by the parameter R, which expresses the increase in variable fluorescence induced by the first flash of a series (F1 – F0) normalized to the maximum variable fluorescence (Fm – F0) measured in the presence of DCMU and HA. The size of the dark- generated ΔpH is calculated for wild-type (WT) (A and C) and ATPsynthase mutant FUD50 (B and D) cells as explained in the text. Broken line, control; solid line, nigericin 10 µM.
Figure 2B shows the kinetics of cyt f re-reduction, measured after a single turnover flash at the same set of luminal pH values. Lowering the pH induced a marked decrease in the rate of cyt f re-reduction, as expected in the case of a reaction (the oxidation of the electron carrier plastoquinol) that couples electron transfer to proton release in the lumen (reviewed in Bendall, 1982). Thus, our experimental system gave us access to two independent reactions that are sensitive to the luminal pH. Their pH sensitivity can be exploited to establish two independent calibration curves that serve to monitor the luminal pH in vivo (Figure 3, squares).
In order to estimate the amplitude of the trans-thylakoid ΔpH existing in dark-adapted algae, we first measured the PSII- and cyt b6f-related reactions in the absence of pH equilibration (no permeant buffer or ionophores added). When analysed with respect to each calibration curve, the two sets of data pointed to the same luminal pH (∼5.5) in the dark-adapted wild type (Figure 3A and C, broken lines). The same procedure was repeated in the presence of 1 µM nigericin (solid lines), which is an H+/K+ ion exchanger that specifically dissipates the trans-thylakoid pH gradient without suppressing the electrical component of Δµ̃H+ (Shavit and San Pietro, 1967). Therefore, nigericin equilibrates the luminal pH with that of the stroma, which has a large buffering capacity. In the absence of permeant buffers, no equilibration between the external medium and the cells was observed upon addition of the ionophore, i.e. no effect of the external pH was observed (data not shown), in agreement with previous reports for C.sorokiniana (Finazzi and Rappaport, 1998). The two calibration curves yield a pH of ∼7 in the presence of nigericin, which reflects the stromal pH in these experimental conditions; this value is in agreement with previous estimations for dark-adapted chloroplasts (Heldt et al., 1973) Thus, a ΔpH of ∼1.5 units builds up in darkness across the thylakoid membranes of dark-grown cells of wild-type C.reinhardtii.
The generation of Δµ̃H+ in C.sorokiniana in darkness has been ascribed to ATP hydrolysis by the membrane-bound ATPsynthase (Rappaport et al., 1999). Therefore, we repeated the same set of PSII- and cyt b6f-sensitive pH measurements using the C.reinhardtii FUD50 mutant that bears a deletion of most of the gene encoding the β subunit and thus has no chloroplast ATPsynthase/ATPase activity (Lemaire et al., 1986). In the presence of nigericin, the mutant had the same characteristics as the wild type with a pH value of 7. However, the pH value remained the same in the absence of protonophore (Figure 3B and D). This experiment indicates that the FUD50 mutant is unable to develop a significant trans-thylakoid ΔpH, thus confirming that the proton source for the ΔpH formed in darkness is the chloroplast ATPsynthase.
Role of trans-thylakoid ΔpH in targeting and accumulation of active PSII and cyt b6f complexes in C.reinhardtii
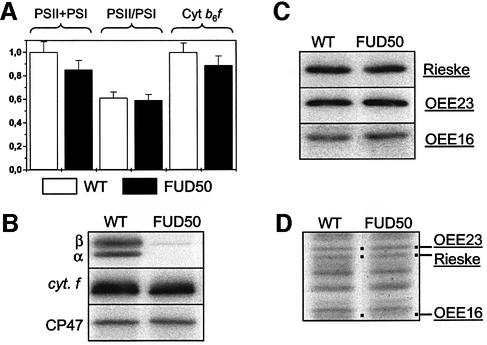
The above study provided the opportunity to investigate the influence of the trans-thylakoid ΔpH on the functional assembly of the photosynthetic electron transfer chain. If it was strictly required for thylakoid targeting of those PSI, PSII and cyt b6f subunits that use the Tat pathway, we would expect the ATPsynthase mutant cells grown in darkness to display large pleiotropic changes in electron transfer reactions and in thylakoid protein accumulation, as in maize mutants defective in the Tat pathway (Voelker and Barkan, 1995). Thus, we compared the total number of active reaction centres, the ratio of PSII to PSI primary and the maximal rate of electron transfer through the b6f complex in the wild type and the FUD50 mutant grown in darkness (Figure 4A). We found no difference between the two, a conclusion further substantiated by the finding that similar amounts of CP47 and cyt f accumulated in their thylakoid membranes whereas, as expected, the major CF1 subunits were missing from the FUD50 mutant (Figure 4B). The three Tat proteins (the Rieske protein, OEE23 and OEE16) accumulated to the same extent in the two dark-grown strains (Figure 4C). In addition, their rate of targeting to the thylakoid membranes, measured as the rate of labelling of the corresponding polypeptides present in membranes purified from dark-grown cells pulse-labelled for 10 min with [14C]acetate, was identical for the two strains (Figure 4D). Thus, thylakoids from the wild type and from FUD50 showed identical protein patterns for the three Tat passenger proteins, an observation that rules out any prominent physiological role for ΔpH in targeting these proteins to their final membrane location.
Fig. 4. Consequences of ΔpH removal by the ATPsynthase mutation FUD50 on protein translocation in the thylakoids of C.reinhardtii. (A) Accumulation of functional PSII and b6f complexes in dark-grown wild-type (WT) and ATPsynthase mutant FUD50 strains. PSI and PSII activities were calculated as described in Materials and methods. Cyt b6f activity was estimated as the rate of cyt f reduction, as in Figure 2. All parameters were normalized to the cellular concentration. (B) Immunodetection of accumulation of α and β subunits of CF1, cyt f and chlorophyll binding protein CP47 of PSII. (C) Immunodetection of accumulation of the Rieske protein of b6f complex and of the oxygen-evolving complex OEE23 and OEE16 subunits of PSII. (D) Autoradiography of membrane-associated protein synthesized during a 10 min pulse in vivo [14C]acetate labelling in the presence of 150 µg/ml chloramphenicol. Tat substrate proteins are underlined, and Sec substrates are in italics. The dots indicate the presence of the protein.
Effect of the H+/K+ exchanger nigericin on protein targeting to thylakoids in vivo
The above results demonstrated that the absence of a ΔpH in the FUD50 mutant had no influence on the efficiency of protein targeting to thylakoids and on protein functional assembly. In order to perform an independent assessment of the role of the trans-thylakoid ΔpH in protein translocation through the Tat pathway in vivo, we looked for the effect of nigericin, which specifically removes the ΔpH component of Δµ̃H+.
Wild-type cells grown in the light were incubated for 1 h in the dark and pulse-labelled for 10 min with [14C]acetate in the absence or presence of nigericin. Again, we found no evidence for a ΔpH-dependent change in the yield of membrane targeting of the Tat passenger proteins whether the effect of nigericin was assessed in the dark (Figure 5) or in the light (data not shown). Similar results were obtained for nigericin concentrations ranging from 1 to 10 µM, an indication that the ionophore concentration was saturating (data not shown). However, we noted that nigericin had a significant effect on the labelling of chloroplast-encoded products (Figure 5, dots), which are identified on the right-hand side of Figure 5. This effect of nigericin probably reflects the requirement of a ΔpH to activate the elongation step of translation, as suggested for the chloroplasts of vascular plants (Mühlbauer and Eichacker, 1998).
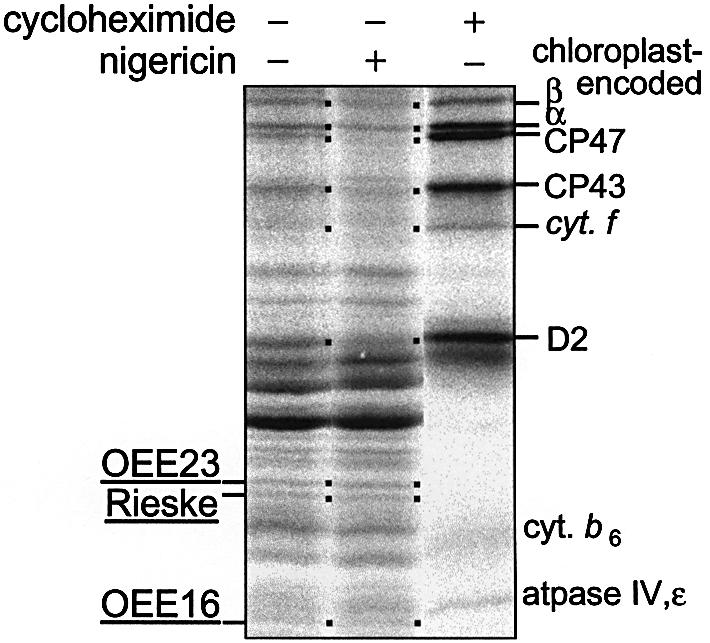
Fig. 5. Consequences of ΔpH removal by the H+/K+ exchanger nigericin on protein translocation in the thylakoids of C.reinhardtii wild-type cells. Autoradiography of membrane-associated protein synthesis during a 10 min in vivo pulse [14C]acetate labelling. Light-grown cells were labelled in the dark in the presence (+) or absence (–) of 10 µM nigericin. The dots indicate predicted protein positions. Addition of 8 µg/ml cycloheximide (an inhibitor of nuclear-encoded protein synthesis) during pulse-labelling is also indicated.
Role of trans-thylakoid ΔpH on the targeting and accumulation of active PSII and cyt b6f complexes in barley leaves
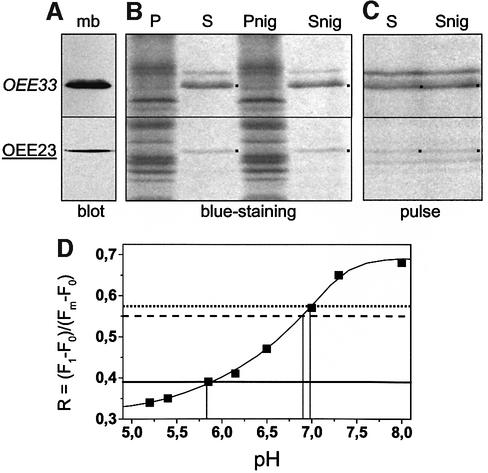
The finding that thylakoid targeting of Tat passenger proteins showed no ΔpH sensitivity in vivo in C.reinhardtii prompted us to perform a similar in vivo experiment with vascular plants. Attached leaves of barley were gently scratched and pulse-labelled for 30 min with droplets of [35S]Cys/Met with and without nigericin. We then compared the rate of labelling of a Sec substrate (OEE33) and a Tat substrate (OEE23), immunodetected in Figure 6A, which we recovered after isolation of thylakoid membranes and extraction with dithiothreitol–Na2CO3 (Figure 6B). Again, the pattern of relative labelling of the two proteins remained similar whether or not the leaves were treated with nigericin (Figure 6C). The ability of nigericin to dissipate the trans-thylakoid ΔpH in intact leaves was confirmed by the same experimental approach as used for C.reinhardtii (Figure 6D); unlike algae, plants fully deactivate the chloroplast ATPsynthase in darkness and therefore display no ΔpH in darkness (broken versus dotted lines). In our experimental conditions, a resting ΔpH of ∼1 unit that remained after illumination of the leaves for 5 min collapsed upon treatment with nigericin (compare solid and dotted lines).
Fig. 6. Consequences of ΔpH removal by the H+/K+ exchanger nigericin on protein translocation in barley leaves. (A) Immunodetection of OEE33 and OEE23 in thylakoid extracted from pulsed leaves (mb). (B) Coomassie Blue staining of proteins of the pellet (P) and supernatant (S) of chaotropic-treated membranes. (C) Autoradiography of membrane-associated protein synthesis during a 30 min pulse [35S]Met/Cys in vivo labelling in the presence or absence of 100 µM nigericin. Tat substrates are underlined and Sec substrates are in italics. (D) Thylakoid membranes uncoupled by nigericin in intact barley leaves. The fluorescence yield is indicated by the parameter R, as in Figure 3. Broken line, dark-adapted sample; solid line, illuminated sample; dotted line, illuminated sample plus nigericin 10 µM. Illumination was followed by a dark adaptation in order to allow re-oxidation of Qa. The calibration curve (squares) is the same when measured in thylakoids isolated from vascular plants (Lavergne and Rappaport, 1998). However, see its close resemblance to that obtained in vivo in C.reinhardtii (Figure 3A).
Discussion
A thylakoid-targeting Tat pathway in C.reinhardtii
Translocation mutants of vascular plants showing severe phenotypic deficiencies have allowed the identification of three thylakoid membrane components of the Tat translocation machinery: cpTatC, Tha4 and Hcf106, which are similar to the Escherichia coli tatC, tatA and tatB gene products, respectively (reviewed in Mori and Cline, 2001; Robinson and Bolhuis, 2001). The C.reinhardtii EST database (http://www.biology.duke.edu/chlamy) contains homologues of cpTatC, Tha4 and Hcf106, which a chloroP algorithm (Emanuelsson et al., 1999) predicts to have chloroplast targeting sequences. Thus, it is reasonable to assume that C.reinhardtii chloroplasts have the same Tat machinery in charge of the same protein substrates as vascular plant chloroplasts. These substrates are identified by the presence of a twin arginine motif in their signal sequence (Teter and Klionsky, 1999; Mori and Cline, 2001; Robinson and Bolhuis, 2001). Among the major Tat substrates are two OEE subunits, OEE23 and OEE16, whose thylakoid targeting sequences are highly conserved between vascular plants and C.reinhardtii chloroplasts. The Tat signature of a third Tat substrate, the Rieske protein, underwent a substitution from RR in bacteria to KR in chloroplasts of vascular plants and algae, but has been shown experimentally by competition experiments to be Tat targeted to the thylakoids with OEE23 (Molik et al., 2001).
In this work, we have tested the relationships between Tat targeting of the Rieske and its conversion to the holoform by altering the endogenous KR motif to a canonical RR motif. Neither thylakoid targeting nor biogenesis of an active Rieske was affected by the substitution. This behaviour excludes the possibility that the KR sequence would be required to slow down the passage of the Rieske protein through the Tat translocon in order to allow the binding of the Fe–S cluster before translocation (Molik et al., 2001). In contrast, conversion of the KR motif to a non-Tat KK motif had dramatic effects on thylakoid targeting, leading to a 70% decrease in the assembly of the Rieske in active cyt b6f complexes. This mutational approach of the Rieske targeting in vivo confirms the role of Tat signalling in the chloroplast of living cells of C.reinhardtii.
Protein translocation by the Tat pathway in vivo
Up to now, most studies of the energetics of protein translocation have been performed in vitro and in organello. These studies have shown conclusively that the Tat pathway does not require ATP (Mould and Robinson, 1991; Cline et al., 1992), but strictly requires a ΔpH (reviewed in Teter and Klionsky, 1999; Berks et al., 2000; Mori and Cline, 2001; Robinson and Bolhuis, 2001). Thus, we were surprised to find that elimination of the trans-thylakoid ΔpH in vivo in C.reinhardtii had no effect on thylakoid targeting of Tat passenger proteins. Previous suggestions for a ΔpH requirement for Tat-mediated protein translocation in vivo came from a limited number of studies with bacteria. Some were based on experiments using carbonyl cyanide m-chlorophenylhydrazone (CCCP) (Santini et al., 1998; Cristóbal et al., 1999), but this ionophore dissipates both the electrical and osmotic components of Δµ̃H+ (e.g. Huang et al., 2002), prevents ATP synthesis and thus affects all translocation pathways (Driessen, 1992; Santini et al., 1998; Cristóbal et al., 1999). Pop et al. (2002) used a heterologous system that consisted of expressing in E.coli a minimum set of proteins of the Tat apparatus from Bacillus subtilis. They observed a nigericin-sensitive export of Tat substrates. This heterologous situation resembles the in vitro/in organello systems if some additional factors are required in vivo to release the ΔpH requirement, as will be discussed below.
One way of reconciling the present data with those obtained in vitro/in organello is to assume that different translocation pathways would substitute for each other better in vivo than in organello or in isolated thylakoids. Indeed, in C.reinhardtii, the decrease in translocation efficiency of plastocyanin upon modification of its Sec presequence was less pronounced in vivo than in vitro (Lawrence and Kindle, 1997). Also, a mutation in the signal sequence of the Sec substrate cyt f inhibits its insertion in the membrane but increases the translocation of Tat substrates in vivo in C.reinhardtii (Smith and Kohorn, 1994). No such substitution mechanisms were reported in vitro (Cline et al., 1993) except for polyphenol oxidase, a protein that may use either the Tat or the Sec pathway (Koussevitzky et al., 1998). Here, we add support to a higher flexibility of the translocation systems in vivo; we observed only a 70% reduction in thylakoid targeting of the KK-addressed Rieske, whereas a similar targeting mutation on OEE23 reduced its rate of thylakoid translocation in isolated chloroplasts by two orders of magnitude (Chaddock et al., 1995).
Despite the above examples, there is no instance where full compensation has been reported in vivo. This would not be easy to reconcile with the phenotype of mutants of the Tat translocon that were isolated from vascular plants, such as the transposon-inserted mutant of TatC of Arabidopsis thaliana (apg2) or the maize mutants tha 4 and hcf106, which show severe pleiotropic defects at the chloroplast level (Voelker and Barkan 1995; Settles et al., 1997; Walker et al., 1999; Motohashi et al., 2001). Thus, our report of a ΔpH insensitivity of the Tat pathway in vivo cannot be ascribed to compensation effects by other routes.
We should consider the validity of our method since measuring a ΔpH in vivo is not an easy task, particularly across an intracellular membrane system. We took advantage of the occurrence of two electron transfer reactions on the lumen side of the thylakoid membranes that are pH sensitive and used them as endogenous (lumen-exposed) pH probes. We found a trans-thylakoid ΔpH of ∼1.5 units established in vivo in the dark using both PSII- and cyt b6f-located reactions as probes. We confirmed that the thylakoid-bound ATPsynthase was the source of proton injection in the luminal compartment in darkness since no ΔpH persisted when a chloroplast ATPsynthase mutant was used instead of the wild type. This mutant showed no alteration in thylakoid targeting of Tat passenger proteins. The ΔpH insensitivity of the Tat pathway in vivo was confirmed in experiments using the protonophore nigericin, which had no effect on the targeting of Tat substrates in either C.reinhardtii cells or attached barley leaves, although this treatment has been previously reported to inhibit protein translocation fully in most of the experiments performed in vitro (reviewed in Teter and Klionsky, 1999; Berks et al., 2000; Mori and Cline, 2001; Robinson and Bolhuis, 2001).
The efficiency of nigericin in dissipating the ΔpH in vivo relative to that observed in vitro could be questioned. It has been suggested that, in some instances, a light-induced ΔpH of ∼0.5 units remains after nigericin treatment of isolated thylakoid membranes (Heldt et al., 1973). The efficiency of proton gradient dissipation by nigericin can only be higher in our experimental conditions since the rate of ΔpH generation in dark-adapted algae is generally slower than that observed upon illumination of isolated thylakoids (for discussion, see Finazzi and Rappaport, 1998). In addition, we have checked that an increase in nigericin concentration by a factor of 10 did not modify the rate of protein import. However, let us assume that a marginal ΔpH persists after nigericin treatment. Then we conclude that the efficiency of protein translocation by the Tat pathway in vivo remains unaltered when ΔpH is reduced from ≥1.5 units to a value <0.5 units. This conclusion is still in marked contrast to what is reported from in vitro experiments, where a large decrease in translocation rate by the Tat pathway was observed upon decreasing ΔpH below 1.5 units (Brock et al., 1995). Indeed, the free energy associated with a ΔpH value of ∼0.5 units would be close to that of thermal fluctuation and therefore far from satisfying the properties of a driving force for protein translocation.
What is the function of a ΔpH dependence of the Tat translocation pathway in vitro?
In our experimental conditions, collapsing the whole Δµ̃H+ leads to an arrest of protein translation because of the fall in intracellular ATP, which precludes further study of protein translocation. Thus, we cannot exclude the possibility that our failure to detect a ΔpH sensitivity of the Tat pathway in vivo could originate from its ability to use ΔΨ as well. This raises the interesting question of the actual energy cost of protein translocation through the Tat pathway, an issue that has rarely been addressed up to now. In one study, Teter and Theg (1998) reported that translocation of Tat substrates across thylakoids did not result in a dissipation of ΔpH. This observation suggested that ΔpH could play an entropic role rather than providing the driving force for protein translocation. In agreement with this view, it was argued (Mori and Cline, 2001, 2002) that ΔpH is involved in the recruitment of one Tat subunit, TatA, to activate the pre-translocon complex made from TatC and TatB. An entropic contribution of ΔpH would easily account for the differences between in vitro and in vivo conditions; the ΔpH sensitivity of the Tat translocation in vitro could arise from the loss of factors, either short lived or loosely bound to the membrane, which participate in vivo in the activation of the Tat translocon. In this view, the efficiency of translocation through the Tat pathway in vivo and in vitro could be very different because the ΔpH activation of translocation may not make up completely for the loss of other translocation cofactors. Indeed, the efficiency of Tat targeting in vitro/in organello is ∼100 times slower than translocation through the Sec pathway (for discussion, see Berks et al., 2000), with a significant fraction of the protein substrates remaining in the stroma, e.g. 70–90% in the case of the Rieske protein (Molik et al., 2001). We found no such evidence in vivo. There is no significant accumulation of stromal intermediates of the Tat substrates, and the rate of labelling of their membrane-bound forms during the time of a pulse relative to that of other thylakoid proteins is not lower than their relative rate of accumulation; this means that their thylakoid targeting rates match the rates of other thylakoid insertion processes.
Several groups have reported the existence of trans-acting proteins for Tat substrates prior to translocation. A peptide leader binding protein was recently identified in E.coli (Oresnik et al., 2001). An azide-sensitive factor was identified for thylakoid targeting of OEE16 (Leheny et al., 1998). In the case of the Rieske protein, stromal interactions with chaperones Cpn60 and Hsp70 have been described (Madueño et al., 1993) and trans-acting factors should participate in Fe2S2 cluster binding, as in the case of bacteria and mitochondria (Mühlenhoff and Lill, 2000). Further biochemical analysis of the translocating substrates of the Tat pathway should enable us to determine whether the contribution of other cofactors has been overlooked.
Materials and methods
Growth conditions
Chlamydomonas reinhardtii strains were grown on Tris–acetate– phosphate (TAP) pH 7.2 at 25°C under continuous illumination at 6 µE/m2/s or in the dark and collected during the exponential phase at 2 × 106 cells/ml. Young seedlings of barley (Hordeum vulgaris) were grown on soil until full development of the second leaf.
Site-directed mutagenesis and nuclear transformation
Plasmid pACR4.5 (ampicillin sensitive/tetracycline resistant) was constructed as described previously (de Vitry et al., 1999) and used as the template for subsequent mutagenesis of petC-RR and petC-KK with oligonucleotides 5′-CTCGTCGGAGGTaCCCGACATGAACAGGCGC AACATCATG-3′ and 5′-CTCGTCGGAGGTaCCCGACATGAACAA GAAGAACATCATG-3′, respectively. Mutant plasmids, detected by restoration of ampicillin resistance, were subsequently screened for the KpnI restriction site, which is underlined in the sequence of oligonucleotides. Chlamydomonas reinhardtii double mutant cells with a cell wall deficiency and a deletion in PETC, referred to here as petC-Δ1 (de Vitry et al., 1999), were transformed as described previously (Kropat et al., 1995) using HindIII-linearized plasmids pACR-RR and pACR-KK. Phototrophic colonies were selected on minimal medium under light intensities of 40–100 µE/m2/s and visible after 2 weeks. Transformants were characterized by restriction analysis of specific PCR-amplified products.
Absorption and fluorescence spectroscopy
Cells were collected and resuspended in HEPES–NaOH 20 mM pH 7.2 in the presence of 10% Ficoll to avoid cell sedimentation. Spectroscopic measurements were performed at room temperature with a home-built spectrophotometer (Joliot and Joliot, 1994). Cyt f reduction was measured as the absorption changed at 554 nm, with a baseline drawn between 545 and 573 nm subtracted (Finazzi et al., 1997). PSI and PSII charge separation was measured as the extent of the electrochromic signal at 515–545 nm, after excitation with a laser pulse at 695 nm. The PSII contribution was deduced as the difference between the signal measured in the absence and the presence of the PSII inhibitor DCMU. Hydroxylamine was added to destroy the manganese cluster responsible for oxygen evolution, and to slow down recombination between the donor and acceptor sides of PSII, which would prevent correct estimation of the PSI/PSII ratio. Fluorescence was measured with the same experimental apparatus, exciting with weak flashes at 480 nm (hitting <1% of the photosynthetic reaction centres) and monitoring the induced fluorescence emission in the near-IR region.
pH equilibration and estimation of ΔpH
When measuring the pH dependence of the cyt b6f turnover rate, algae were incubated for 30 min with permeant buffers (Na acetate 30 mM in the pH 4.5–6 range or Na imidazole 30 mM in the pH 6–7.5 range). These buffers were required to achieve equilibration between the external medium and the cell cytoplasm (for further discussion, see Finazzi and Rappaport, 1998). Small amounts (1 µM) of the ionophore FCCP were added to facilitate pH equilibration between the cellular compartments. At this low concentration, the ionophore allowed equilibration of the internal compartments of the cells without inducing the modifications of the mechanism of the catalytic cycle of the cytochrome b6f complex that are observed at higher concentrations (Barbagallo et al., 2000). Ficoll (10% w/v) was also added to avoid cell sedimentation. This procedure is essentially the same as that employed in the case of C.sorokiniana (Finazzi and Rappaport, 1998), except that imidazole was preferred to phosphate in the pH 6–7.5 range as it proved to be more effective with C.reinhardtii. In the case of the pH calibration of chlorophyll fluorescence, similar results were obtained when pH was imposed either by the addition of permeant buffers or by cell permeabilization through p-benzoquinone treatments (Rappaport et al., 1999).
In vivo labelling, isolation, separation and analysis of thylakoid proteins
Whole cells of C.reinhardtii were pulse-labelled with [14C]acetate for 10 min under illumination of intensity 50 µE/m/s or in the dark (Lemaire et al., 1986). Thylakoid membranes were purified as described previously (Breyton et al., 1994). Attached second leaves of barley were pulse-labelled with [35S]Met/Cys for 30 min at a light intensity of 50 µE/m2/s (Barkan, 1998), and frozen in liquid nitrogen before preparation of thylakoid membranes as described previously (Jennings et al., 1983). Scratching the leaf proved most efficient for homogeneous drug penetration, as demonstrated by measurements with a fluorescence video imaging system of the increase in fluorescence upon addition of DCMU. Polypeptide DTT–Na2CO3 extraction was carried out as described previously (Breyton et al., 1994). Polypeptides were separated on 12–18% SDS–polyacrylamide gels containing 8 M urea (Lemaire et al., 1986). Immunodetection used antisera raised against subunits of ATPsynthase (α, β), PSII (CP47, OEE23, OEE16), LHCIIP11 and cyt b6f (cyt f, Rieske protein) of C.reinhardtii and 125I-labelled protein A, or against PSII (OEE33, OEE23) of vascular plants and the enhanced chemiluminescence peroxidase method (Breyton et al., 1994).
Acknowledgments
Acknowledgements
We thank Toivo Kallas for help with Rieske mutagenesis, Dominique Drapier for isolation of FUD50 clones, Yves Pierre for C.reinhardtii Rieske antisera, Anna Sokolenko for plant OEE33 and OEE23 antisera, Elisabetta Bergantino for advice with plant labelling, and Fabrice Rappaport and Yves Choquet for discussions and critical reading of the manuscript. This work was supported by the CNRS (UPR1261), the CNR (I.B.) and an Erasmus fellowship to C.C.
References
- Amin P., Sy,D.A.C., Pilgrim,M.L., Parry,D.H., Nussaume,L. and Hoffman,N.E. (1999) Arabidopsis mutants lacking the 43- and 54-kilodalton subunits of the chloroplast signal recognition particle have distinct phenotypes. Plant Physiol., 121, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo R.P, Breyton,C. and Finazzi,G. (2000) Kinetic effects of the electrochemical proton gradient on plastoquinone reduction at the Qi site of the cytochrome b6f complex. J. Biol. Chem., 275, 26121–26127. [DOI] [PubMed] [Google Scholar]
- Barkan A. (1998) Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol., 297, 38–56. [Google Scholar]
- Bendall D. (1982) Photosynthetic cytochromes of oxygenic organisms. Biochim. Biophys. Acta, 683, 119–151. [Google Scholar]
- Bennoun P. (1970) Reoxidation of the fluorescence quencher ‘Q’ in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Biochim. Biophys. Acta, 216, 357–363. [DOI] [PubMed] [Google Scholar]
- Bennoun P. (1982) A respiratory chain in the thylakoid membranes of Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA, 79, 4352–4356. [Google Scholar]
- Berks B.C., Sargent,F. and Palmer,T. (2000) The Tat protein export. Mol. Microbiol., 35, 260–274. [DOI] [PubMed] [Google Scholar]
- Breyton C., de Vitry,C. and Popot,J.L. (1994) Membrane association of cytochrome b6f subunits. The Rieske protein of Chlamydomonas reinhardtii is an extrinsic protein. J. Biol. Chem., 269, 7597–7602. [PubMed] [Google Scholar]
- Brock I.W., Mills,J.D., Robinson,D. and Robinson,C. (1995) The ΔpH-driven, ATP-independent protein translocation mechanism in the chloroplast thylakoid membrane. Kinetics and energetics. J. Biol. Chem., 270, 1657–1662. [DOI] [PubMed] [Google Scholar]
- Chaddock A.M., Mant,A., Karnauchov,I., Brink,S., Herrmann,R.G., Klösgen,R.B. and Robinson,C. (1995) A new type of signal peptide: central role of a twin-arginine motif in transfer signals for the ΔpH dependent thylakoidal protein translocase. EMBO J., 14, 2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.A. and Theg,S.M. (1997) A folded protein can be transported across the chloroplast envelope and thylakoid membranes. Mol. Biol. Cell, 8, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Ettinger,W.F. and Theg,S.M. (1992) Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J. Biol. Chem., 267, 2688–2696. [PubMed] [Google Scholar]
- Cline K., Henry,R., Li,C. and Yuan,J. (1993) Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J., 12, 4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristóbal S., de Gier,J.W., Nielsen,H. and von Heijne,G. (1999) Competition between Sec- and TAT-dependent protein translocation in Escherichia coli. EMBO J., 18, 2982–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry C., Olive,J., Drapier,D., Recouvreur,M. and Wollman,F.A. (1989) Posttranslational events leading to the assembly of photosystem II protein complex: a study using photosynthesis mutants from Chlamydomonas reinhardtii. J. Cell Biol., 109, 991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry C., Finazzi,G. Baymann,F. and Kallas,T. (1999) Analysis of the nucleus-encoded and chloroplast-targeted Rieske protein by classic and site-directed mutagenesis of Chlamydomonas. Plant Cell, 11, 2031–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A.J.M. (1992) Bacterial protein translocation-kinetic and thermodynamic role of ATP and protonmotive force. Trends Biochem. Sci., 17, 219–223. [DOI] [PubMed] [Google Scholar]
- Duysens L.M.N. and Sweers,H.E. (1963) Mechanism of two photochemical reactions in algae as studied by means of fluorescence. In Japanese Society of Plant Physiologists (eds), Microalgae and Photosynthetic Bacteria. University of Tokyo Press, Tokyo, Japan, pp. 353–372.
- Emanuelsson O., Nielsen,H. and von Heijne,G. (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci., 8, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst F., Hoffschulte,H.K., Thome-Kromer,B., Swidersky,U.E., Werner,P.K. and Muller,M. (1994) Precursor-specific requirements for SecA, SecB, and ΔµH+ during protein export of Escherichia coli. J. Biol. Chem., 269, 12840–12845. [PubMed] [Google Scholar]
- Finazzi G. and Rappaport,F. (1998) In vivo characterization of the electrochemical proton gradient generated in darkness in green algae and its kinetic effects on cytochrome b6f turnover. Biochemistry, 37, 9999–10005. [DOI] [PubMed] [Google Scholar]
- Finazzi G., Buschlen,S., de Vitry,C., Rappaport,F., Joliot,P. and Wollman,F.A. (1997) Function-directed mutagenesis of the cytochrome b6f complex in Chlamydomonas reinhardtii: involvement of the cd loop of cytochrome b6 in quinol binding to the Qo site. Biochemistry, 36, 2867–2874. [DOI] [PubMed] [Google Scholar]
- Heldt H.W., Werdan,K., Milovancev,M. and Geller,G. (1973) Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim. Biophys. Acta, 314, 224–241. [DOI] [PubMed] [Google Scholar]
- Huang S., Ratliff,K.S. and Matouschek,A. (2002) Protein unfolding by the mitochondrial membrane potential. Nat. Struct. Biol., 9, 301–307. [DOI] [PubMed] [Google Scholar]
- Hynds P.J., Robinson,D. and Robinson,C. (1998) The Sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J. Biol. Chem., 273, 34868–34874. [DOI] [PubMed] [Google Scholar]
- Jennings R.C., Garlaschi,F.M. and Gerola,P.D. (1983) A study on the lateral distribution of the plastoquinone pool with respect to the photosystem II in stacked and unstacked spinach cloroplastst. Biochim. Biophys. Acta, 722, 144–149. [Google Scholar]
- Joliot P. and Joliot,A. (1994) Mechanism of electron transfer in the bf complex of algae: evidence for a semiquinone cycle. Proc. Natl Acad. Sci. USA, 91, 1034–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouranov A. and Schnell,D.J. (1996) Protein translocation at the envelope and thylakoid membranes of chloroplasts. J. Biol. Chem., 271, 31009–31012. [DOI] [PubMed] [Google Scholar]
- Koussevitzky S., Ne’eman,E., Sommer,A., Steffens,J.C. and Harel,E. (1998) Purification and properties of a novel chloroplast stromal peptidase: processing of polyphenol oxidase and other imported precursors. J. Biol. Chem., 273, 27064–27069. [DOI] [PubMed] [Google Scholar]
- Kropat J., von Gromoff,E.D., Müller,F.W. and Beck,C.F. (1995) Heat shock and light activation of a Chlamydomonas HSP70 gene are mediated by independent regulatory pathways. Mol. Gen. Genet., 248, 727–734. [DOI] [PubMed] [Google Scholar]
- Lavergne J. and Rappaport,F. (1998) Stabilization of charge separation and photochemical misses in photosystem II. Biochemistry, 37, 7899–7906. [DOI] [PubMed] [Google Scholar]
- Lawrence S.D. and Kindle,K.L. (1997) Alterations in the Chlamydomonas plastocyanin transit peptide have distinct effects on in vitro import and in vivo protein accumulation. J. Biol. Chem., 272, 20357–20363. [DOI] [PubMed] [Google Scholar]
- Leheny E.A., Teter,S.A. and Theg,M. (1998) Identification of a role for an azide-sensitive factor in the thylakoid transport of the 17-kilodalton subunit of the photosynthetic oxygen-evolving complex. Plant Physiol., 116, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire C., Girard-Bascou,J., Wollman,F.A. and Bennoun,P. (1986) Studies on the cytochrome b6f complex. I. Characterization of the complex subunits in Chlamydomonas reinhardtii. Biochim. Biophys. Acta, 851, 229–238. [Google Scholar]
- Madueño F., Napier,J.A. and Gray,J.C. (1993) Newly imported Rieske iron–sulfur protein associates with both Cpn60 and Hsp70 in the chloroplast stroma. Plant Cell, 5, 1865–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madueño F., Bradshaw,S.A. and Gray,J.C. (1994) The thylakoid-targeting domain of the chloroplast Rieske iron-sulfur protein is located in the N-terminal hydrophobic region of the mature protein. J. Biol. Chem., 269, 17458–17463. [PubMed] [Google Scholar]
- Mant A., Schmidt,I., Herrmann,R.G., Robinson,C. and Klosgen,R.B. (1995) Sec-dependent thylakoid protein translocation. ΔpH requirement is dictated by passenger protein and ATP concentration. J. Biol. Chem., 270, 23275–23281. [DOI] [PubMed] [Google Scholar]
- Michl D., Robinson,C., Shackleton,J.B., Herrmann,R.G. and Klösgen,R.B. (1994) Targeting of proteins to the thylakoids by bipartite sequences: CF0II is imported by a novel third pathway. EMBO J., 13, 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molik S., Karnauchov,I., Weidlich,C., Herrmann,R.G. and Klosgen,R.B. (2001) The Rieske FeS protein of the cytochrome b6f complex in chloroplasts: missing link in the evolution of protein transport pathways in chloroplasts? J. Biol. Chem., 276, 42761–42766. [DOI] [PubMed] [Google Scholar]
- Mori H. and Cline,K. (2001) Post-translational protein translocation into thylakoids by the Sec and ΔpH-dependent pathways. Biochim. Biophys. Acta, 1541, 80–90. [DOI] [PubMed] [Google Scholar]
- Mori H. and Cline,K. (2002) A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid ΔpH/Tat translocase. J. Cell Biol., 157, 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi R., Nagata,N., Ito,T., Takahashi,S., Hobo,T., Yoshida,S. and Shinozaki,K. (2001) An essential role of a TatC homologue of a ΔpH-dependent protein transporter in thylakoid membrane formation during chloroplast development in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA, 98, 10499–10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould R.M. and Robinson,C. (1991) A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J. Biol. Chem., 266, 12189–12193. [PubMed] [Google Scholar]
- Mühlbauer S.K. and Eichacker,L.A. (1998) Light-dependent formation of the photosynthetic proton gradient regulates translation elongation in chloroplasts. J. Biol. Chem., 273, 20935–20940. [DOI] [PubMed] [Google Scholar]
- Mühlenhoff U. and Lill,R. (2000) Biogenesis of iron–sulfur proteins in eukaryotes: a novel task of mitochondria that is inherited from bacteria. Biochim. Biophys. Acta, 1459, 370–382. [DOI] [PubMed] [Google Scholar]
- Oresnik I.J., Ladner,C.L. and Turner,R.J. (2001) Identification of a twin-arginine leader binding protein. Mol. Microbiol., 40, 323–331. [DOI] [PubMed] [Google Scholar]
- Pfanner N. and Geisler,A. (2001) Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell Biol., 2, 339–349. [DOI] [PubMed] [Google Scholar]
- Pop O., Martin,U., Abel,C. and Muller,J.P. (2002) The twin-arginine signal peptide of PhoD and the TatAd/Cd proteins of Bacillus subtilis form an autonomous Tat translocation system. J. Biol. Chem., 277, 3268–3273. [DOI] [PubMed] [Google Scholar]
- Rappaport F., Finazzi,G., Pierre,Y. and Bennoun,P. (1999) A new electrochemical gradient generator in thylakoid membranes of green algae. Biochemistry, 38, 2040–2047. [DOI] [PubMed] [Google Scholar]
- Reumann S. and Keegstra,K. (1999) The endosymbiotic origin of the protein import machinery of chloroplastic envelope membranes. Trends Plant Sci., 4, 302–307. [DOI] [PubMed] [Google Scholar]
- Robinson C. and Bolhuis,A. (2001) Protein targeting by the twin-arginine translocation pathway. Nat. Rev. Mol. Cell Biol., 2, 350–356. [DOI] [PubMed] [Google Scholar]
- Santini C.L., Ize,B., Chanal,A., Müller,M., Giordano,G. and Wu,L.F. (1998) A novel Sec-independent perisplasmic protein translocation pathway in Escherichia coli. EMBO J., 17, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini C.L., Bernadac,A., Zhang,M., Chanal,A., Ize,B., Blanco,C. and Wu,L.F. (2001) Translocation of jellyfish green fluorescent protein via the Tat system of Escherichia coli and change of periplasmic localization in response to osmotic up-shock. J. Biol. Chem., 276, 8159–8164. [DOI] [PubMed] [Google Scholar]
- Settles A.M., Yonetani,A., Baron,A., Bush,D.R., Cline,K. and Martienssen,R. (1997) Sec-independent protein translocation by the maize Hcf106 protein. Science, 278, 1467–1470. [DOI] [PubMed] [Google Scholar]
- Shavit N. and San Pietro,A. (1967) K+ dependent uncoupling of photophosphorylation by nigericin. Biochem. Biophys. Res. Commun., 28, 277–283. [DOI] [PubMed] [Google Scholar]
- Smith T. and Kohorn,B.D. (1994) Mutations in a signal sequence for the thylakoid membrane identify multiple protein pathways and nuclear suppressors. J. Cell Biol., 126, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter S.A. and Klionsky,D.J. (1999) How to get a folded protein across a membrane. Trends Cell Biol., 9, 428–431. [DOI] [PubMed] [Google Scholar]
- Teter S.A. and Theg,S.M. (1998) Energy-transducing thylakoid membranes remain highly impermeable to ions during protein translocation. Proc. Natl Acad. Sci. USA, 95, 1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.D., Daniel,R.A., Errington,J. and Robinson,C. (2001) Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol., 39, 47–52. [DOI] [PubMed] [Google Scholar]
- Voelker R. and Barkan,A. (1995) Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J., 14, 3905–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.B., Roy,L.M., Coleman,E., Voelker,R. and Barkan,A. (1999) The maize tha4 gene functions in sec-independent protein transport in chloroplasts and is related to hcf106, tatA, and tatB. J. Cell Biol., 147, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F.A., Minai,L. and Nechushtai,R. (1999) The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim. Biophys. Acta, 1411, 21–85. [DOI] [PubMed] [Google Scholar]
- Yuan J. and Cline,K. (1994) Plastocyanin and the 33-kDa subunit of the oxygen-evolving complex are transported into thylakoids with similar requirements as predicted from pathway specificity. J. Biol. Chem., 269, 18463–18467. [PubMed] [Google Scholar]