Abstract
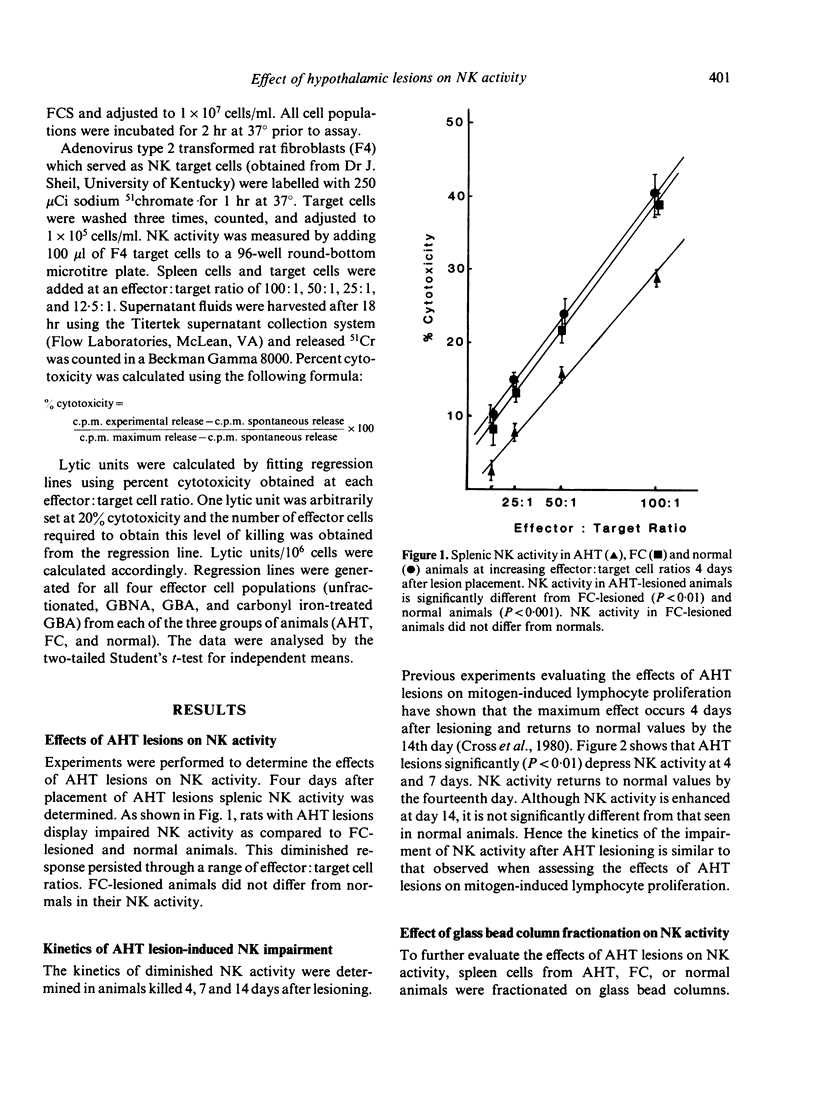
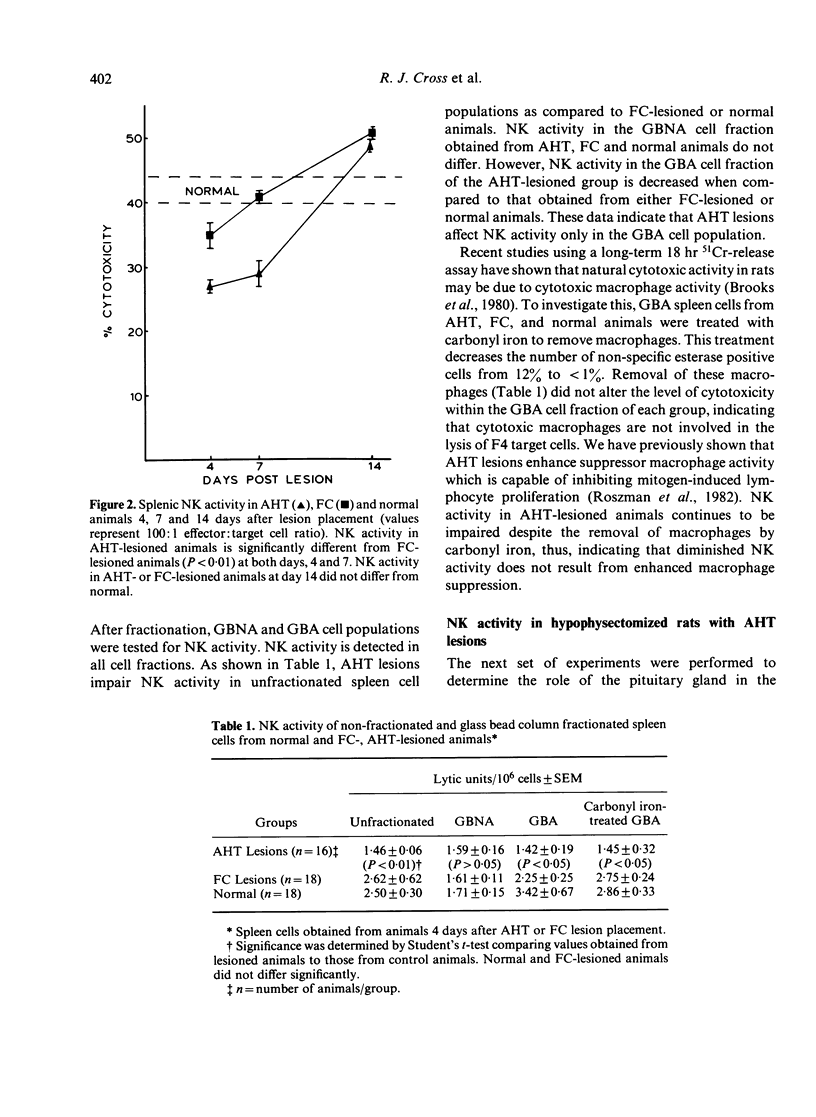
Placement of bilateral electrolytic lesions in the preoptic-anterior hypothalamic area (AHT) of Fischer 344 rats results in decreased splenic NK activity as compared to control and normal animals. Animals with AHT lesions have a decrease in NK activity 4 and 7 days after lesion placement, with a return to normal activity by day 14. Fractionation of spleen cells on glass bead columns results in nonadherent and adherent cell fractions with NK activity. AHT lesions affect NK activity only in the adherent cell fraction. The removal of macrophages from this cell fraction did not restore NK activity. Moreover, this NK activity is not the result of cytotoxic macrophages. Hypophysectomy decreases NK activity in lesioned and non-lesioned animals, suggesting the influence of pituitary factors. These data indicate that the anterior hypothalamus is capable of modulating the cytotoxic activity of NK cells. Thus, neuroimmunomodulation may be a potential factor in susceptibility to some disease states such as viral infections and neoplasia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose C. T. The essential role of corticosteroids in the induction of the immune response in vitro. Ciba Found Study Group. 1970;36:100–125. [PubMed] [Google Scholar]
- Bartrop R. W., Luckhurst E., Lazarus L., Kiloh L. G., Penny R. Depressed lymphocyte function after bereavement. Lancet. 1977 Apr 16;1(8016):834–836. doi: 10.1016/s0140-6736(77)92780-5. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A., Sorkin E., Da Prada M., Keller H. H. Immunoregulation mediated by the sympathetic nervous system. Cell Immunol. 1979 Dec;48(2):346–355. doi: 10.1016/0008-8749(79)90129-1. [DOI] [PubMed] [Google Scholar]
- Besedovsky H., Sorkin E. Network of immune-neuroendocrine interactions. Clin Exp Immunol. 1977 Jan;27(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- Bliznakov E. G. Serotonin and its precursors as modulators of the immunological responsiveness in mice. J Med. 1980;11(2-3):81–105. [PubMed] [Google Scholar]
- Brooks C. G., Flannery G. R., Webb P. J., Baldwin R. W. Quantitative studies of natural immunity to solid tumours in rats. The nature of the killer cell depends on the type of assay. Immunology. 1980 Nov;41(3):673–680. [PMC free article] [PubMed] [Google Scholar]
- Brooks W. H., Cross R. J., Roszman T. L., Markesbery W. R. Neuroimmunomodulation: neural anatomical basis for impairment and facilitation. Ann Neurol. 1982 Jul;12(1):56–61. doi: 10.1002/ana.410120111. [DOI] [PubMed] [Google Scholar]
- Brooks W. H., Netsky M. G., Normansell D. E., Horwitz D. A. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972 Dec 1;136(6):1631–1647. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. J., Brooks W. H., Roszman T. L., Markesbery W. R. Hypothalamic-immune interactions. Effect of hypophysectomy on neuroimmunomodulation. J Neurol Sci. 1982 Mar;53(3):557–566. doi: 10.1016/0022-510x(82)90250-7. [DOI] [PubMed] [Google Scholar]
- Cross R. J., Markesbery W. R., Brooks W. H., Roszman T. L. Hypothalamic-immune interactions. I. The acute effect of anterior hypothalamic lesions on the immune response. Brain Res. 1980 Aug 25;196(1):79–87. doi: 10.1016/0006-8993(80)90717-9. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY T. F., BERLINER M. L., SCHNEEBELI G. L., BERLINER D. L. HORMONAL CONTROL OF LYMPHATIC STRUCTURE AND FUNCTION. Ann N Y Acad Sci. 1964 Feb 28;113:825–843. doi: 10.1111/j.1749-6632.1964.tb40707.x. [DOI] [PubMed] [Google Scholar]
- Dann J. A., Wachtel S. S., Rubin A. L. Possible involvement of the central nervous system in graft rejection. Transplantation. 1979 Apr;27(4):223–226. doi: 10.1097/00007890-197904000-00002. [DOI] [PubMed] [Google Scholar]
- Devoino L., Eliseeva L., Eremina O., Idova G., Cheido M. 5-Hydroxytryptophan effect on the development of the immune response: IgM and IgG antibodies and rosette formation in primary and secondary responses. Eur J Immunol. 1976 Jun;5(6):394–399. doi: 10.1002/eji.1830050608. [DOI] [PubMed] [Google Scholar]
- Fabris N. Immunodepression in thyroid-deprived animals. Clin Exp Immunol. 1973 Dec;15(4):601–611. [PMC free article] [PubMed] [Google Scholar]
- Fabris N., Pierpaoli W., Sorkin E. Hormones and the immunological capacity. IV. Restorative effects of developmental hormones or of lymphocytes on the immunodeficiency syndrome of the dwarf mouse. Clin Exp Immunol. 1971 Aug;9(2):227–240. [PMC free article] [PubMed] [Google Scholar]
- Filipp G., Mess B. Role of the thyroid hormone system in suppression of anaphylaxis due to electrolytic lesion of the tuberal region of the hypothalamus. Ann Allergy. 1969 Oct;27(10):500–505. [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Gilman S. C., Schwartz J. M., Milner R. J., Bloom F. E., Feldman J. D. beta-Endorphin enhances lymphocyte proliferative responses. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4226–4230. doi: 10.1073/pnas.79.13.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron L. T., Jr, Crutcher K. A., Davis J. N. Lymph nodes--a possible site for sympathetic neuronal regulation of immune responses. Ann Neurol. 1980 Nov;8(5):520–525. doi: 10.1002/ana.410080509. [DOI] [PubMed] [Google Scholar]
- Goldowitz D., Scheff S. W., Cotman C. W. The specificity of reactive synaptogenesis: a comparative study in the adult rat hippocampal formation. Brain Res. 1979 Jul 20;170(3):427–441. doi: 10.1016/0006-8993(79)90962-4. [DOI] [PubMed] [Google Scholar]
- Hanna N., Schneider M. Enhancement of tumor metastasis and suppression of natural killer cell activity by beta-estradiol treatment. J Immunol. 1983 Feb;130(2):974–980. [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Hochman P. S., Cudkowicz G., Evans P. D. Carrageenan-induced decline of natural killer activity. II. Inhibition of cytolysis by adherent non-T La-negative suppressor cells activated in vivo. Cell Immunol. 1981 Jun;61(1):200–201. doi: 10.1016/0008-8749(81)90366-x. [DOI] [PubMed] [Google Scholar]
- Hoffman P. M., Robbins D. S., Nolte M. T., Gibbs C. J., Jr, Gajdusek D. C. Cellular immunity in Guamanians with amyotrophic lateral sclerosis and Parkinsonism-dementia. N Engl J Med. 1978 Sep 28;299(13):680–685. doi: 10.1056/NEJM197809282991302. [DOI] [PubMed] [Google Scholar]
- Isaković K., Janković B. D. Neuro-endocrine correlates of immune response. II. Changes in the lymphatic organs of brain-lesioned rats. Int Arch Allergy Appl Immunol. 1973;45(3):373–384. doi: 10.1159/000231055. [DOI] [PubMed] [Google Scholar]
- Janković B. D., Isaković K. Neuro-endocrine correlates of immune response. I. Effects of brain lesions on antibody production, Arthus reactivity and delayed hypersensitivity in the rat. Int Arch Allergy Appl Immunol. 1973;45(3):360–372. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Johnson H. M., Smith E. M., Torres B. A., Blalock J. E. Regulation of the in vitro antibody response by neuroendocrine hormones. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4171–4174. doi: 10.1073/pnas.79.13.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K., Tanaka S., Ito T., Hamashima Y. Suppression of the primary immune response by chemical sympathectomy. Res Commun Chem Pathol Pharmacol. 1977 Apr;16(4):687–694. [PubMed] [Google Scholar]
- LUPARELLO T. J., STEIN M., PARK C. D. EFFECT OF HYPOTHALAMIC LESIONS ON RAT ANAPHYLAXIS. Am J Physiol. 1964 Oct;207:911–914. doi: 10.1152/ajplegacy.1964.207.4.911. [DOI] [PubMed] [Google Scholar]
- Martz E. Mechanism of specific tumor-cell lysis by alloimmune T lymphocytes: resolution and characterization of discrete steps in the cellular interaction. Contemp Top Immunobiol. 1977;7:301–361. doi: 10.1007/978-1-4684-3054-7_9. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O. Regulation of the immune response by the major histocompatibility system. N Engl J Med. 1980 Dec 25;303(26):1514–1517. doi: 10.1056/NEJM198012253032606. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Maestroni G. J. Pharmacological control of the hormonally modulated immune response. II. Blockade of antibody production by a combination of drugs acting on neuroendocrine functions. Its prevention by gonadotropins and corticotrophin. Immunology. 1978 Mar;34(3):419–430. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Maestroni G. J. Pharmacological control of the immune response by blockade of the early hormonal changes following antigen injection. Cell Immunol. 1977 Jun 15;31(2):355–363. doi: 10.1016/0008-8749(77)90037-5. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN A. F., Jr, SPENCER E. S., MARSH J. T. Decrease in susceptibility of mice to passive anaphylaxis following avoidance-learning stress. Proc Soc Exp Biol Med. 1959 Apr;100(4):878–879. doi: 10.3181/00379727-100-24811. [DOI] [PubMed] [Google Scholar]
- Rasmussen A. F., Jr Emotions and immunity. Ann N Y Acad Sci. 1969 Oct 14;164(2):458–462. doi: 10.1111/j.1749-6632.1969.tb14060.x. [DOI] [PubMed] [Google Scholar]
- Roszman T. L., Cross R. J., Brooks W. H., Markesbery W. R. Hypothalamic-immune interactions. II. The effect of hypothalamic lesions on the ability of adherent spleen cells to limit lymphocyte blastogenesis. Immunology. 1982 Apr;45(4):737–742. [PMC free article] [PubMed] [Google Scholar]
- Saxena Q. B., Saxena R. K., Adler W. H. Regulation of natural killer activity in vivo. III. Effect of hypophysectomy and growth hormone treatment on the natural killer activity of the mouse spleen cell population. Int Arch Allergy Appl Immunol. 1982;67(2):169–174. [PubMed] [Google Scholar]
- Solomon G. F. Emotions, stress, the central nervous system, and immunity. Ann N Y Acad Sci. 1969 Oct 14;164(2):335–343. doi: 10.1111/j.1749-6632.1969.tb14048.x. [DOI] [PubMed] [Google Scholar]
- Solomon G. F. Stress and antibody response in rats. Int Arch Allergy Appl Immunol. 1969;35(1):97–104. doi: 10.1159/000230163. [DOI] [PubMed] [Google Scholar]
- Tyrey L., Nalbandov A. V. Influence of anterior hypothalamic lesions on circulating antibody titers in the rat. Am J Physiol. 1972 Jan;222(1):179–185. doi: 10.1152/ajplegacy.1972.222.1.179. [DOI] [PubMed] [Google Scholar]
- Vischer T. L. Effect of hydrocortisone on the reactivity of thymus and spleen cells of mice to in vitro stimulation. Immunology. 1972 Nov;23(5):777–784. [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Felten D. L. Sympathetic innervation of murine thymus and spleen: a comparative histofluorescence study. Anat Rec. 1981 Apr;199(4):531–542. doi: 10.1002/ar.1091990409. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Peterson R. G., Shea P. A., Schmedtje J. F., Bauer D. C., Felten D. L. Sympathetic innervation of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res Bull. 1981 Jan;6(1):83–94. doi: 10.1016/s0361-9230(81)80072-x. [DOI] [PubMed] [Google Scholar]
- Williams R. M., Leifer J., Moore M. J. Hybrid effect in natural cell-mediated cytotoxicity of SV40-transformed fibroblasts by rat spleen cells. Transplantation. 1977 Mar;23(3):283–286. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


