Abstract
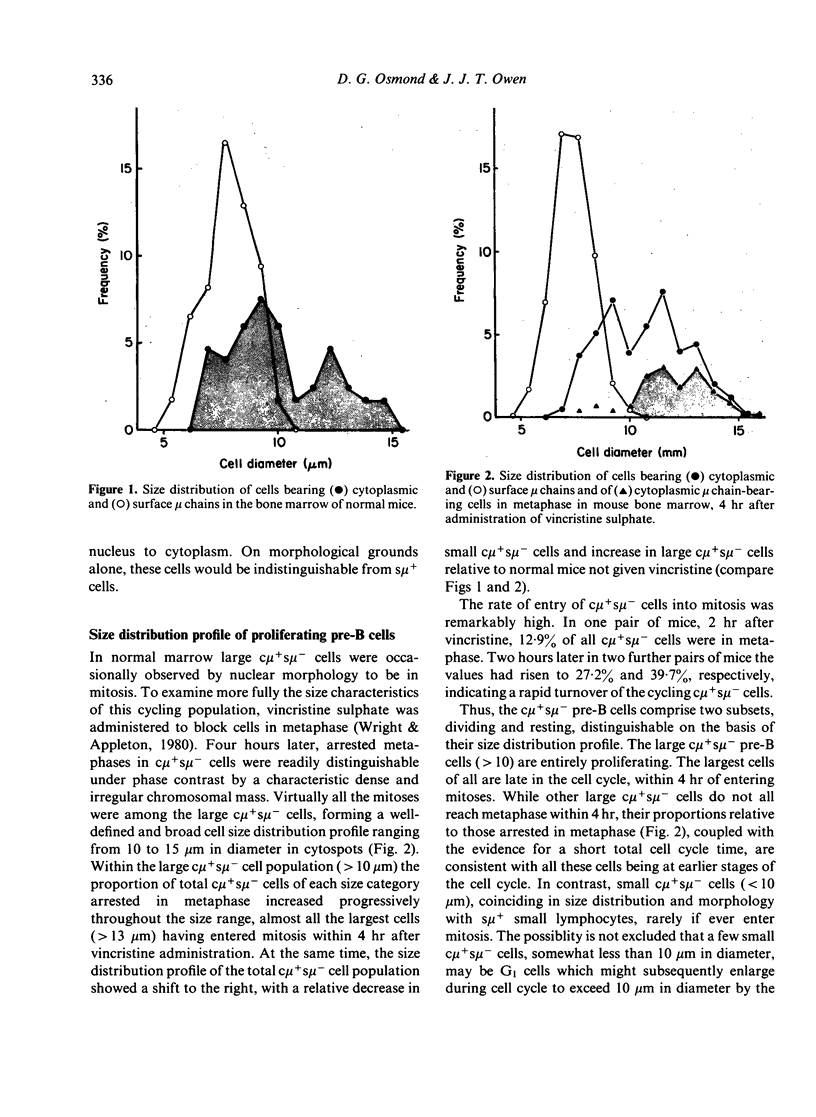
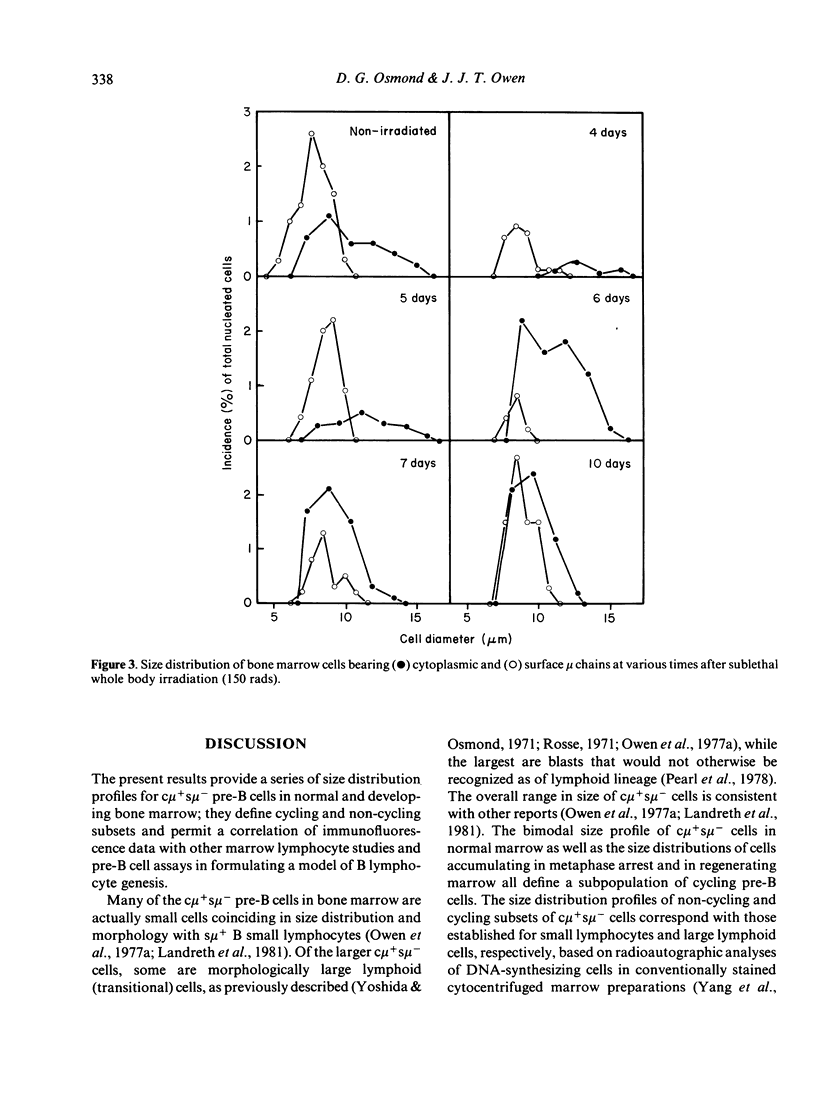
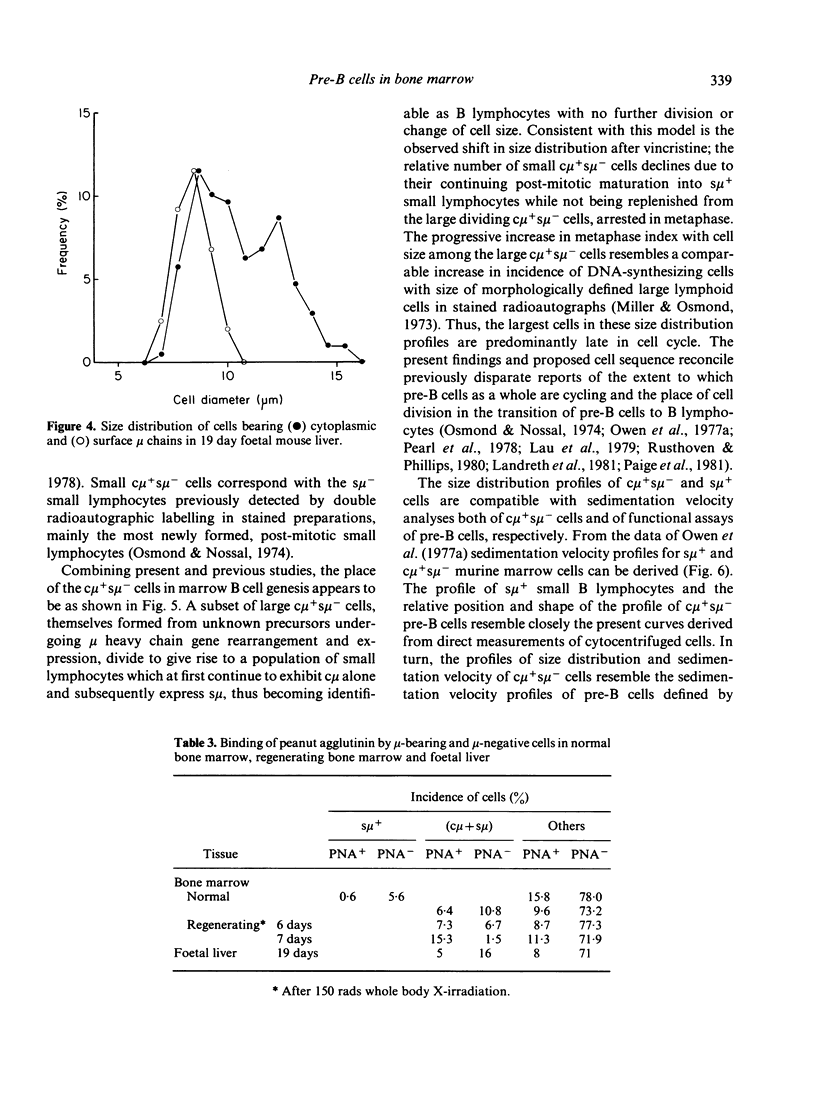
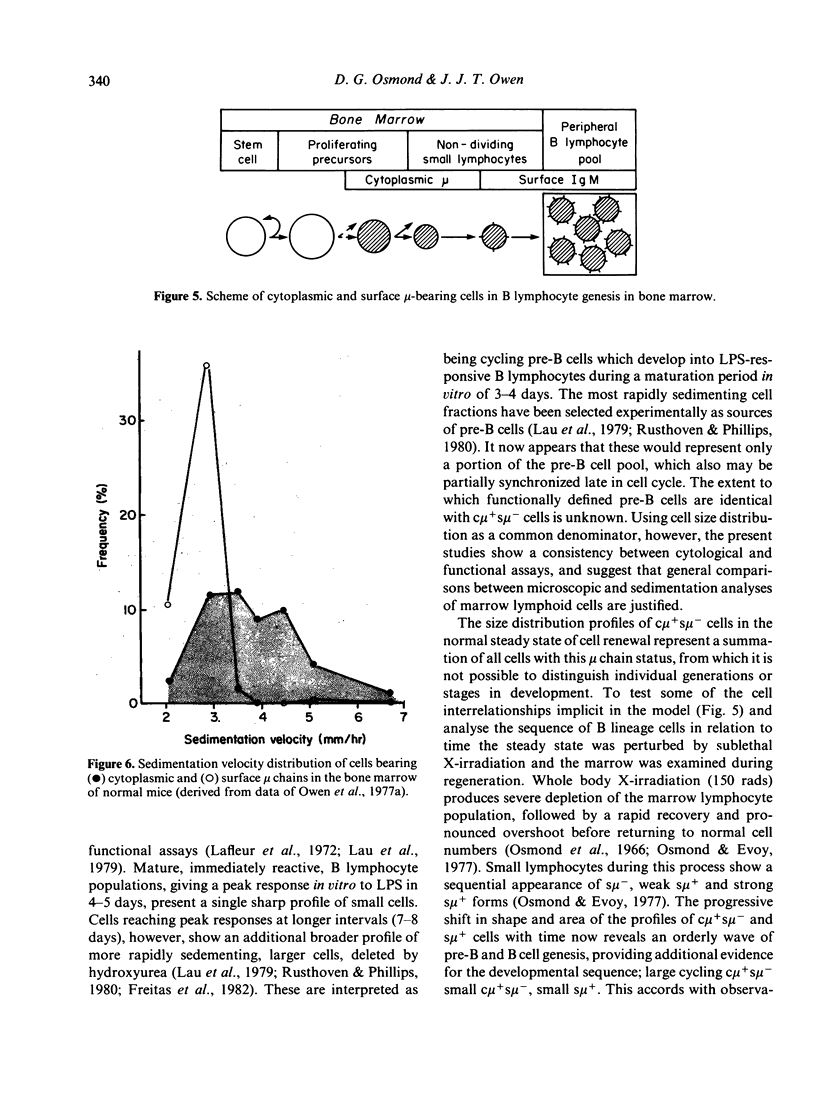
Pre-B cell populations in mouse bone marrow, identified by double immunofluorescence labelling of cytoplasmic and surface mu chains (c mu, s mu), have been characterized by cell size, proliferative capacity and the binding of peanut agglutinin (PNA). In the normal steady state of lymphocyte production the size distribution profile of cytocentrifuged c mu + s mu- cells was bimodal. A population of large cells in rapid cell cycle was revealed by arresting cells in mitosis with vincristine. Many c mu + s mu- cells, however, formed a nondividing population of small lymphocytes, resembling s mu + cells in size distribution. During regeneration from sublethal whole body X-irradiation (150 rads) a marked enrichment of large c mu + s mu- cells preceded small c mu + s mu- and s mu + cells; progressive changes in cell size distribution reflected a wave of B lymphocyte genesis. The c mu + s mu- cells in foetal liver resembled those in regenerating marrow. Surface binding of PNA characterised all c mu + s mu- cell populations in normal and regenerating bone marrow and in foetal liver, whereas only a minority of s mu + cells and mu-negative marrow cells bound PNA strongly. The present size distribution analyses allow a correlation with other cytological and functional studies of marrow lymphocyte precursors in defining the place of pre-B cells in B lymphocyte genesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahim F., Osmond D. G. Migration of bone marrow lymphocytes demonstrated by selective bone marrow labeling with thymidine-H3. Anat Rec. 1970 Oct;168(2):139–159. doi: 10.1002/ar.1091680202. [DOI] [PubMed] [Google Scholar]
- Burrows P. D., Kearney J. F., Lawton A. R., Cooper M. D. Pre-B cells: bone marrow persistence in anti-mu-suppressed mice, conversion to B lymphocytes, and recovery after destruction by cyclophosphamide. J Immunol. 1978 May;120(5):1526–1531. [PubMed] [Google Scholar]
- Freitas A. A., Rocha B., Forni L., Coutinho A. Population dynamics of B lymphocytes and their precursors: demonstration of high turnover in the central and peripheral lymphoid organs. J Immunol. 1982 Jan;128(1):54–60. [PubMed] [Google Scholar]
- Lafleur L., Miller R. G., Phillips R. A. A quantitative assay for the progenitors of bone marrow-associated lymphocytes. J Exp Med. 1972 Jun 1;135(6):1363–1374. doi: 10.1084/jem.135.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth K. S., Rosse C., Clagett J. Myelogenous production and maturation of B lymphocytes in the mouse. J Immunol. 1981 Nov;127(5):2027–2034. [PubMed] [Google Scholar]
- Lau C. Y., Melchers F., Miller R. G., Phillips R. A. In vitro differentiation of B lymphocytes from pre-B cells. J Immunol. 1979 Apr;122(4):1273–1277. [PubMed] [Google Scholar]
- Melchers F. B lymphocyte development in fetal liver. II. Frequencies of precursor B cells during gestation. Eur J Immunol. 1977 Jul;7(7):482–486. doi: 10.1002/eji.1830070715. [DOI] [PubMed] [Google Scholar]
- Miller S. C., Osmond D. G. The proliferation of lymphoid cells in guinea-pig bone marrow. Cell Tissue Kinet. 1973 May;6(3):259–269. doi: 10.1111/j.1365-2184.1973.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Boss M. A. Expression of binding sites for peanut agglutinin during murine B lymphocyte differentiation. Immunology. 1980 Jun;40(2):193–200. [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Staber F. G., Johnson G. R., Metcalf D., Battye F. L. Differential expression of lectin receptors during hemopoietic differentiation: enrichment for granulocyte-macrophage progenitor cells. J Cell Physiol. 1980 May;103(2):217–237. doi: 10.1002/jcp.1041030207. [DOI] [PubMed] [Google Scholar]
- Osmond D. G., Nossal G. J. Differentiation of lymphocytes in mouse bone marrow. II. Kinetics of maturation and renewal of antiglobulin-binding cells studied by double labeling. Cell Immunol. 1974 Jul;13(1):132–145. doi: 10.1016/0008-8749(74)90233-0. [DOI] [PubMed] [Google Scholar]
- Osmond D. G., Roylance P. J., Lee W. R., Ramsell T. G., Yoffey J. M. The effect of unilateral limb shielding on the haemopoietic response of the guinea-pig to gamma irradiation (150 r.). Br J Haematol. 1966 Jul;12(4):365–375. doi: 10.1111/j.1365-2141.1966.tb05646.x. [DOI] [PubMed] [Google Scholar]
- Owen J. J., Wright D. E., Habu S., Raff M. C., Cooper M. D. Studies on the generation of B lymphocytes in fetal liver and bone marrow. J Immunol. 1977 Jun;118(6):2067–2072. [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Shinefeld L. A., Sato V. L. Precursors of murine B lymphocytes. Physical and functional characterization, and distinctions from myeloid stem cells. J Exp Med. 1981 Jan 1;153(1):154–165. doi: 10.1084/jem.153.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl E. R., Vogler L. B., Okos A. J., Crist W. M., Lawton A. R., 3rd, Cooper M. D. B lymphocyte precursors in human bone marrow: an analysis of normal individuals and patients with antibody-deficiency states. J Immunol. 1978 Apr;120(4):1169–1175. [PubMed] [Google Scholar]
- Raff M. C., Megson M., Owen J. J., Cooper M. D. Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature. 1976 Jan 22;259(5540):224–226. doi: 10.1038/259224a0. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Rose M. L., Birbeck M. S., Wallis V. J., Forrester J. A., Davies A. J. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980 Mar 27;284(5754):364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- Rosse C. Small lymphocyte and transitional cell populations of the bone marrow; their role in the mediation of immune and hemopoietic progenitor cell functions. Int Rev Cytol. 1976;45:155–290. doi: 10.1016/s0074-7696(08)60080-7. [DOI] [PubMed] [Google Scholar]
- Rusthoven J. J., Phillips R. A. Hydroxyurea kills B cell precursors and markedly reduces functional B cell activity in mouse bone marrow. J Immunol. 1980 Feb;124(2):781–786. [PubMed] [Google Scholar]
- Wright N. A., Appleton D. R. The metaphase arrest technique. A critical review. Cell Tissue Kinet. 1980 Nov;13(6):643–663. doi: 10.1111/j.1365-2184.1980.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Yang W. C., Miller S. C., Osmond D. G. Maturation of bone marrow lymphocytes. II. Development of Fc and complement receptors and surface immunoglobulin studied by rosetting and radioautography. J Exp Med. 1978 Nov 1;148(5):1251–1270. doi: 10.1084/jem.148.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]


