Abstract
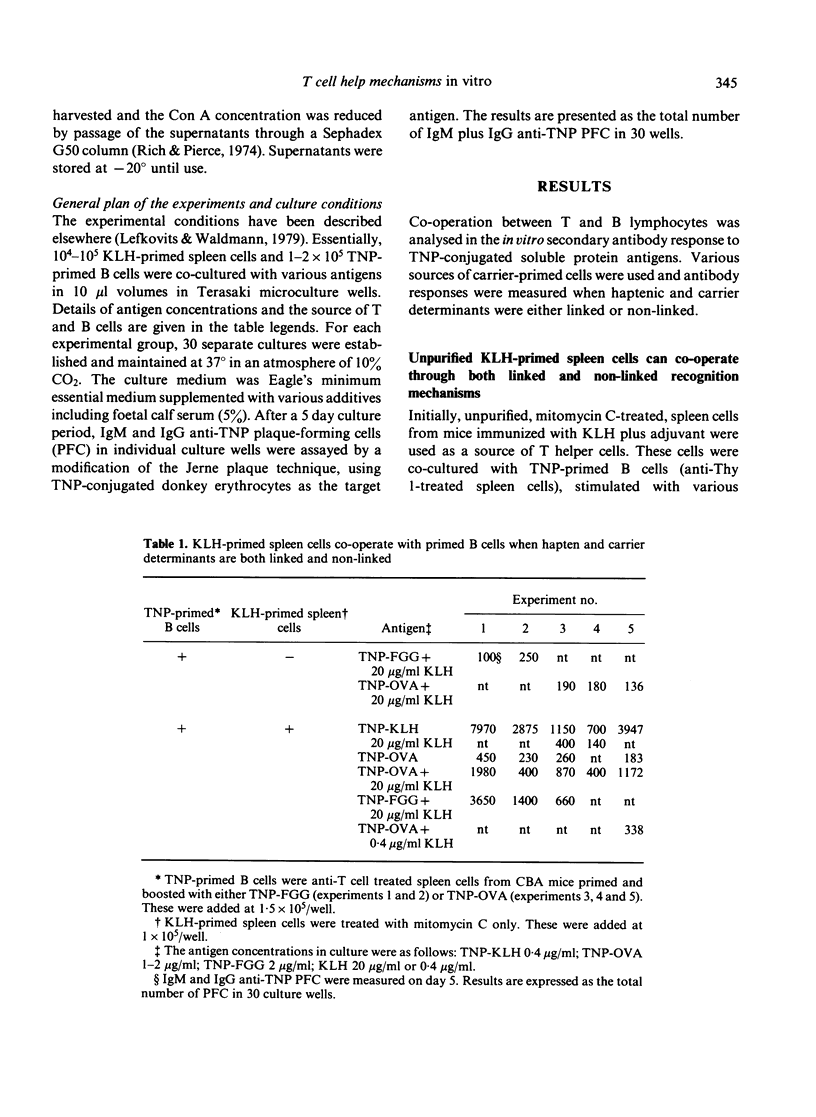
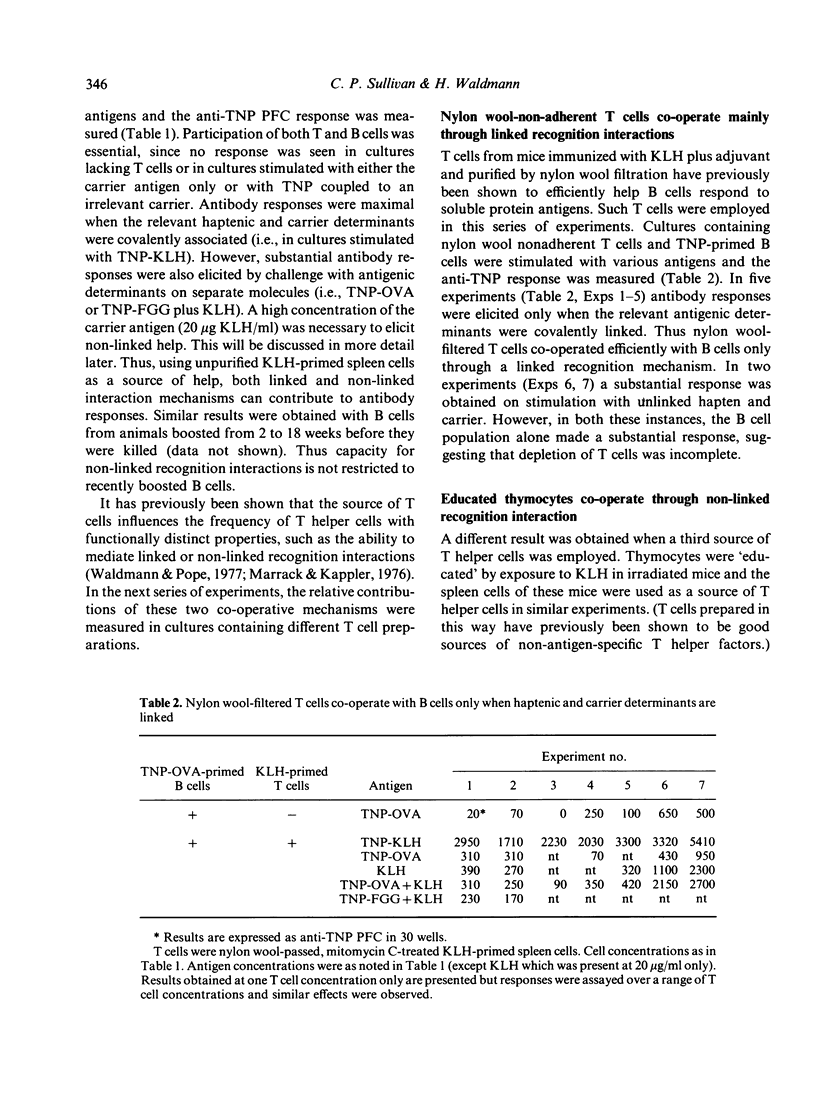
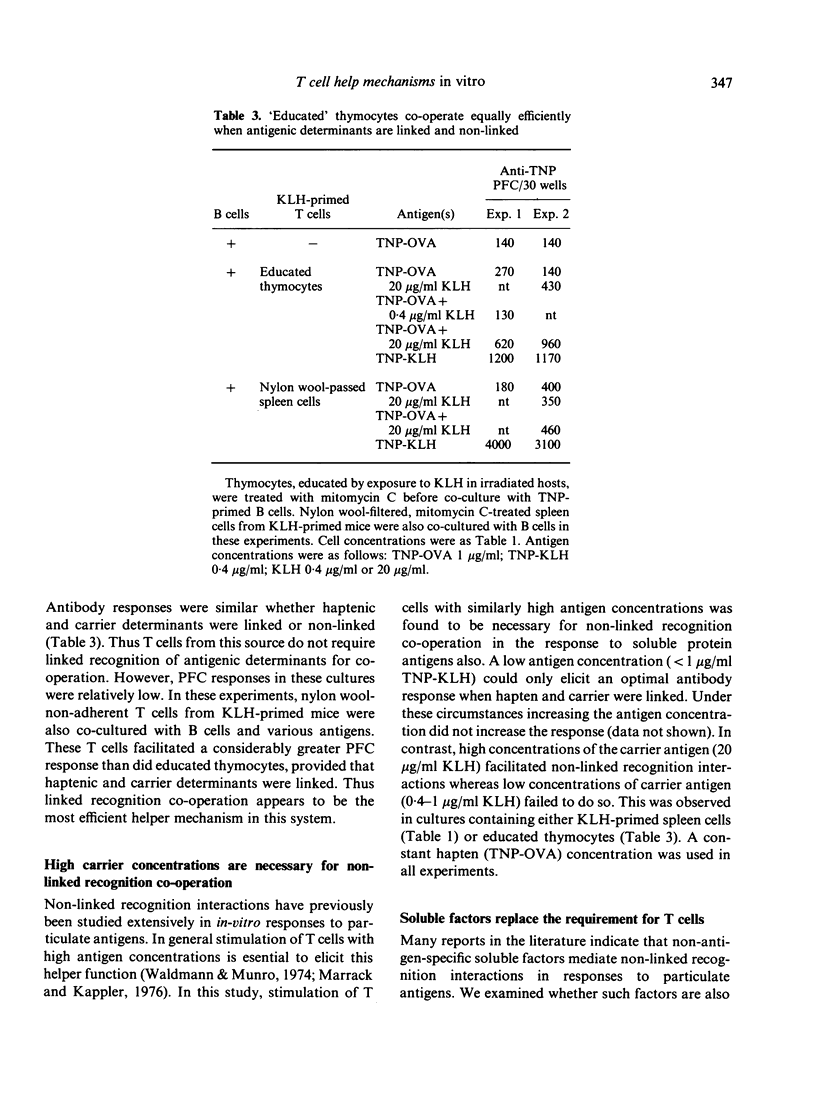
Mechanisms by which T and B lymphocytes co-operate in the in vitro secondary antibody response to trinitrophenyl (TNP)-conjugated soluble protein antigens were investigated. The generation of antibody responses was analyzed when haptenic and carrier determinants were either linked or non-linked. Ability to co-operate through each of these mechanisms was influenced by the experimental conditions employed, particularly the mode of preparation of the T cells and the antigen concentration used. Nylon wool filtration of T cells may deplete a T helper population involved in non-linked recognition interactions. High antigen concentrations favour the non-linked form of interaction whereas low antigen concentrations favour linked recognition interaction. These data suggest that at least two distinct co-operative mechanisms co-exist. However, experimental conditions can be defined under which either one mechanism predominates or where more than one mechanism is relevant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Schreier M. H., Melchers F. T-cell-dependent B-cell stimulation is H-2 restricted and antigen dependent only at the resting B-cell level. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1612–1616. doi: 10.1073/pnas.77.3.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Sorvillo J., Mecarelli-Halbwachs L., Leibowitch J. Mechanism of action of the C4 nephritic factor. Deregulation of the classical pathway of C3 convertase. J Exp Med. 1981 Jul 1;154(1):1–12. doi: 10.1084/jem.154.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell L., Kappler J. W., Marrack P. Antigen-specific and nonspecific mediators of T cell/B cell cooperation. III. Characterization of the nonspecific mediator(s) from different sources. J Immunol. 1976 May;116(5):1379–1384. [PubMed] [Google Scholar]
- Henry C. In vitro anti-hapten response to a lactoside-conjugated protein. Cell Immunol. 1975 Sep;19(1):117–128. doi: 10.1016/0008-8749(75)90296-8. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Keller D. M., Swierkosz J. E., Marrack P., Kappler J. W. Two types of functionally distinct, synergizing helper T cells. J Immunol. 1980 Mar;124(3):1350–1359. [PubMed] [Google Scholar]
- Kishimoto T., Ishizaka K. Regulation of antibody response in vitro. VII. Enhancing soluble factors for IgG and IgE antibody response. J Immunol. 1973 Oct;111(4):1194–1205. [PubMed] [Google Scholar]
- Marrack P., Kappler J. W. Antigen-specific and nonspecific mediatiors of T cell/B cell cooperation. II. Two helper T cells distinguished by their antigen sensitivities. J Immunol. 1976 May;116(5):1373–1378. [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular cooperation. Eur J Immunol. 1971 Jan;1(1):18–27. doi: 10.1002/eji.1830010104. [DOI] [PubMed] [Google Scholar]
- North J. R., Kemshead J. T., Askonas B. A. Non-specific factor replaces T cells in an IgG response to soluble antigens. Immunology. 1977 Sep;33(3):321–329. [PMC free article] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. 3. Suppression of plaque-forming cell responses in vitro by supernatant fluids from concanavalin A-activated spleen cell cultures. J Immunol. 1974 Apr;112(4):1360–1368. [PubMed] [Google Scholar]
- Rittenberg M. B., Amkraut A. A. Immunogenicity of trinitrophenyl-hemocyanin: production of primary and secondary anti-hapten precipitins. J Immunol. 1966 Sep;97(3):421–430. [PubMed] [Google Scholar]
- Schimpl A., Wecker E., Hünig T. Mechanism of T-cell help in the immune response to soluble protein antigens. I. Evidence for in situ generation and action of T-cell-replacing factor during the anamnestic response to dinitrophenyl keyhole limpet hemocyanin in vitro. J Exp Med. 1977 May 1;145(5):1216–1227. doi: 10.1084/jem.145.5.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Stimulation of IgG antibody response in vitro by T cell-replacing factor. J Exp Med. 1973 Feb 1;137(2):547–552. doi: 10.1084/jem.137.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Takemori T., Okumura K., Nonaka M., Tokuhisa T. Two distinct types of helper T cells involved in the secondary antibody response: independent and synergistic effects of Ia- and Ia+ helper T cells. J Exp Med. 1978 Feb 1;147(2):446–458. doi: 10.1084/jem.147.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). I. Functional characterization of a TRF-producing helper T cell subset and genetic studies on TRF production. J Immunol. 1980 May;124(5):2414–2422. [PubMed] [Google Scholar]
- Waldmann H., Munro A. Letter: T cell-dependent mediator in the immune response. Nature. 1973 Jun 8;243(5406):356–357. doi: 10.1038/243356a0. [DOI] [PubMed] [Google Scholar]
- Waldmann H., Pope H. The influence of antigen presentation on the generation of T-helper cells with different functions. Immunology. 1977 Nov;33(5):721–725. [PMC free article] [PubMed] [Google Scholar]
- Waldmann H. T cell-dependent mediator in the immune response. III. The role of non-specific factor (NSF) in the in vitro immune response. Immunology. 1975 Mar;28(3):497–507. [PMC free article] [PubMed] [Google Scholar]


