Abstract
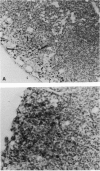
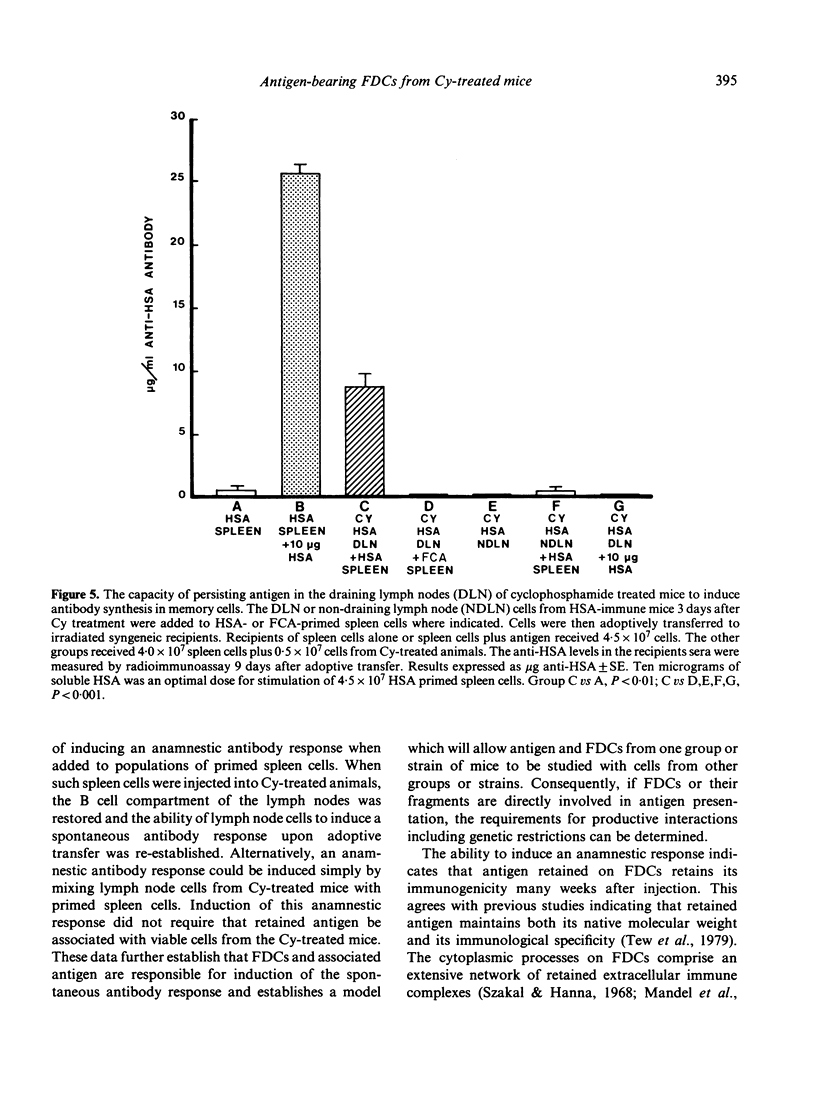
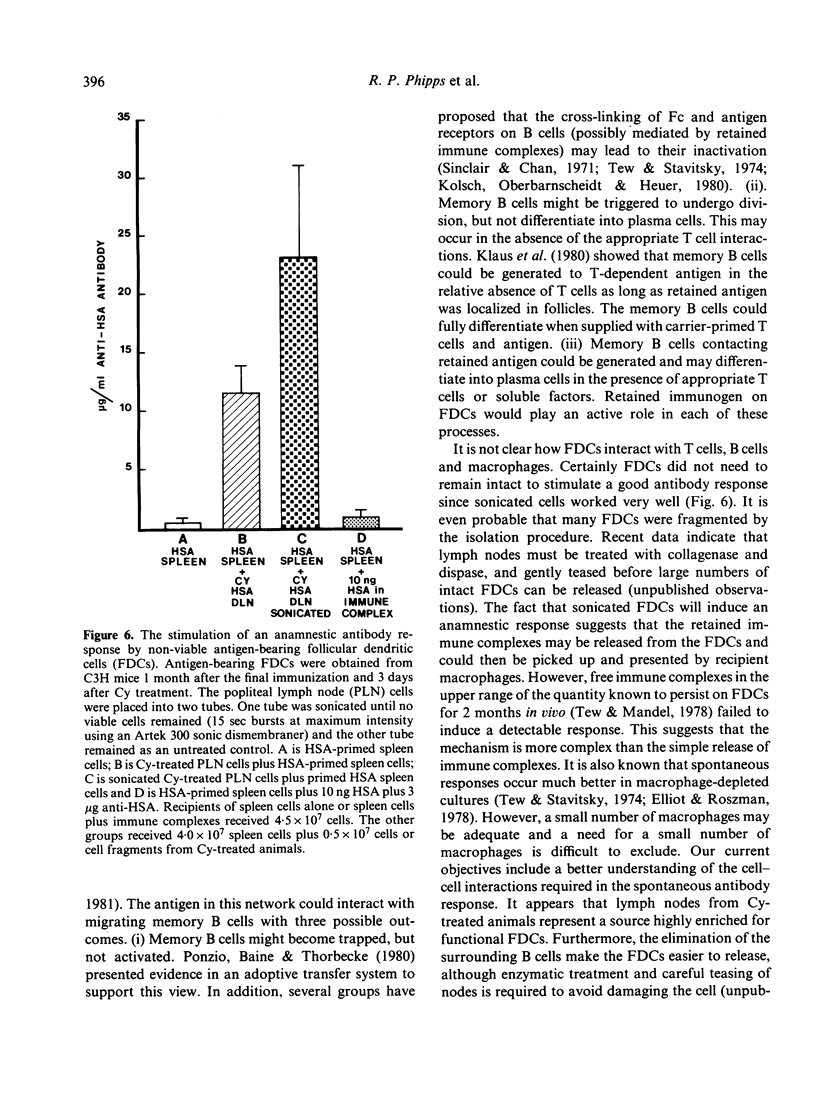
In contrast to most mouse lymph node cells, follicular dendritic cells (FDCs) resist cyclophosphamide (Cy; 300 mg/kg)-mediated destruction in vivo. In this study we sought to determine if antigen-bearing FDCs from Cy-treated animals maintained biological activity. We were especially interested in whether FDCs from Cy-treated animals could stimulate an antibody response when combined with primed spleen cells and whether the FDCs needed to be intact and viable for stimulation to occur. The effect of Cy treatment on lymph node histology, number of T cells and B cells, and the 'spontaneous antibody response' was determined. Cy treatment resulted in a massive depletion of the lymph node cortex and a loss of follicles and germinal centres. Over 90% of B cells in the lymph node were eliminated. The paracortex was more resistant although nearly 80% of T cells were eliminated. Cy treatment also eliminated the 'spontaneous antibody response' as established by in vitro culture or after adoptive transfer. The addition of primed spleen cells to antigen-bearing FDCs including sonicated non-viable FDCs from Cy-treated animals resulted in an anamnestic antibody response. Memory lymphocytes, injected into the hind foot pads of Cy-treated animals, migrated to the follicular area of popliteal lymph nodes and cells from these reconstituted nodes spontaneously responded upon subsequent adoptive transfer. It was concluded that antigen retained on Cy-treated FDCs maintains its immunogenicity and is capable of inducing a 'spontaneous antibody response' or an anamnestic response. Furthermore antigen on FDCs or on fragments of FDCs from one animal can interact with memory cells from another animal to induce a productive antibody response. Lymph nodes enriched for FDCs by Cy treatment should be a good source of FDCs for isolation and further study of the nature of this interaction.
Full text
PDF




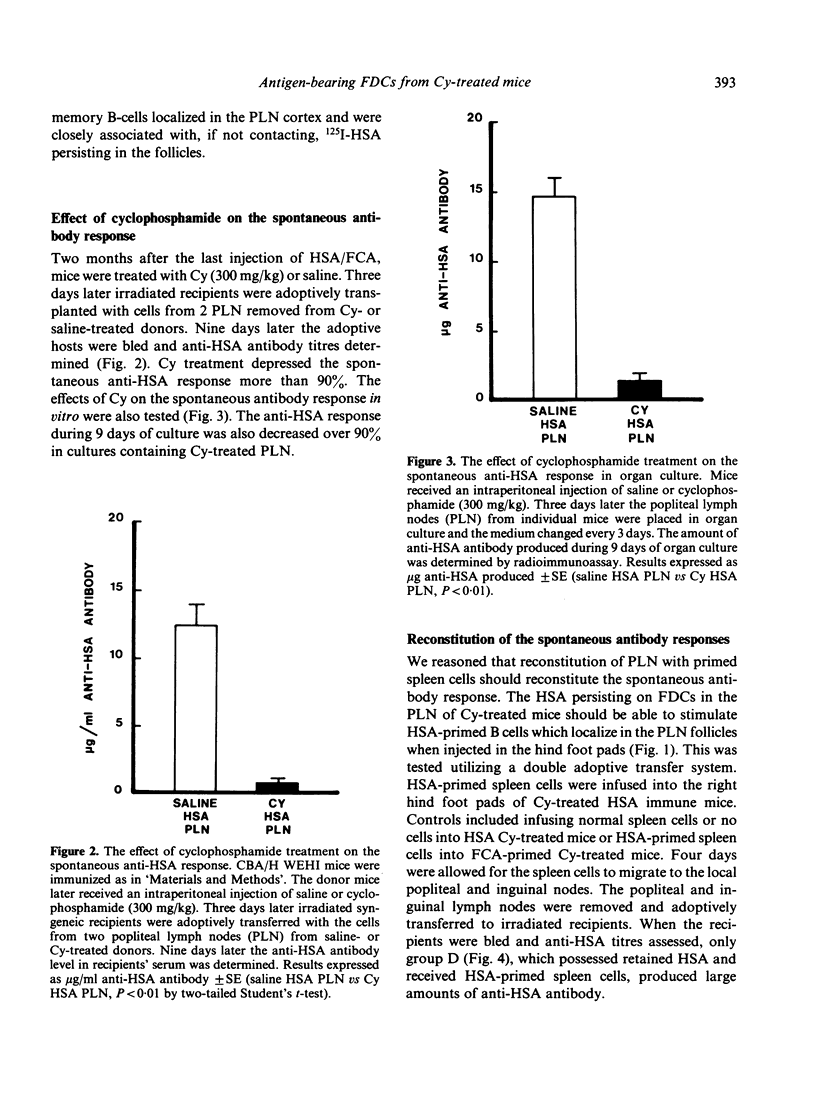
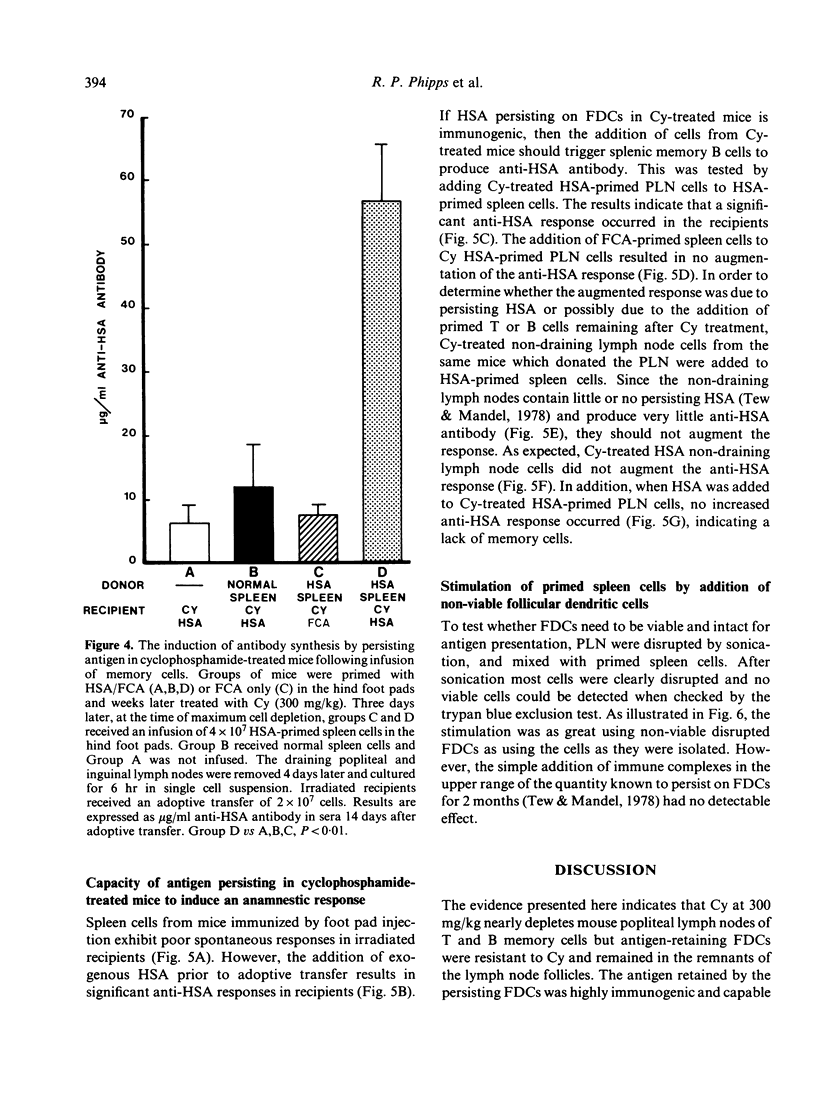

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elliott L. H., Roszman T. L. Suppressor cell regulation of the spontaneously induced in vitro secondary antibody response. Cell Immunol. 1978 Sep 15;40(1):46–57. doi: 10.1016/0008-8749(78)90314-3. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Humphrey J. H., Kunkl A., Dongworth D. W. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3–28. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Mandel T. E., Kennedy M. M. The differentiation of murine thymocytes in vivo and in vitro. Immunology. 1978 Aug;35(2):317–331. [PMC free article] [PubMed] [Google Scholar]
- Mandel T. E., Phipps R. P., Abbot A., Tew J. G. The follicular dendritic cell: long term antigen retention during immunity. Immunol Rev. 1980;53:29–59. doi: 10.1111/j.1600-065x.1980.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Peeters S. H., Carter B. G. Regulation of the IgE antibody response in mice. I. Long-term production of IgE Anti-ovalbumin antibody in irradiated recipients. J Immunol. 1978 Oct;121(4):1596–1602. [PubMed] [Google Scholar]
- Phipps R. P., Mandel T. E., Tew J. G. Effect of immunosuppressive agents on antigen retained in lymphoid follicles and collagenous tissue of immune mice. Cell Immunol. 1981 Jan 15;57(2):505–516. doi: 10.1016/0008-8749(81)90108-8. [DOI] [PubMed] [Google Scholar]
- Phipps R. P., Tew J. G., Miller G. A., Mandel T. E. A murine model for analysis of spontaneous induction and feedback regulation of specific antibody synthesis. Immunol Commun. 1980;9(1):55–70. doi: 10.3109/08820138009050806. [DOI] [PubMed] [Google Scholar]
- Schmidtke J. R., Unanue E. R. Macrophage-antigen interaction: uptake, metabolism and immunogenicity of foreign albumin. J Immunol. 1971 Aug;107(2):331–338. [PubMed] [Google Scholar]
- Stecher V. J., Thorbecke G. J. Gamma globulin and antibody formation in vitro. VII. Effect of x-irradiation on the secondary antibody response in vitro. J Exp Med. 1967 Jan 1;125(1):33–44. doi: 10.1084/jem.125.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakal A. K., Hanna M. G., Jr The ultrastructure of antigen localization and viruslike particles in mouse spleen germinal centers. Exp Mol Pathol. 1968 Feb;8(1):75–89. doi: 10.1016/0014-4800(68)90007-5. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. E., Burgess A. W. Retention of intact HSA for prolonged periods in the popliteal lymph nodes of specifically immunized mice. Cell Immunol. 1979 Jun;45(1):207–212. doi: 10.1016/0008-8749(79)90378-2. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. The maintenance and regulation of serum antibody levels: evidence indicating a role for antigen retained in lymphoid follicles. J Immunol. 1978 Mar;120(3):1063–1069. [PubMed] [Google Scholar]
- Tew J. G., Phipps R. P., Mandel T. E. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Self C. H., Harold W. W., Stavitsky A. B. The spontaneous induction of anamnestic antibody synthesis in lymph node cell cultures many months after primary immunization. J Immunol. 1973 Aug;111(2):416–423. [PubMed] [Google Scholar]
- Tew J. G., Stavitsky A. B. In vitro studies linking induction of spontaneous antibody synthesis to an accessory cell with persisting antigen on its surface. Cell Immunol. 1974 Oct;14(1):1–11. doi: 10.1016/0008-8749(74)90163-4. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Shortman K. The separation of different cell classes from lymphoid organs. IX. A simple and rapid method for removal of damaged cells from lymphoid cell suspensions. J Immunol Methods. 1973 Apr;2(3):293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]