Abstract
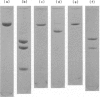
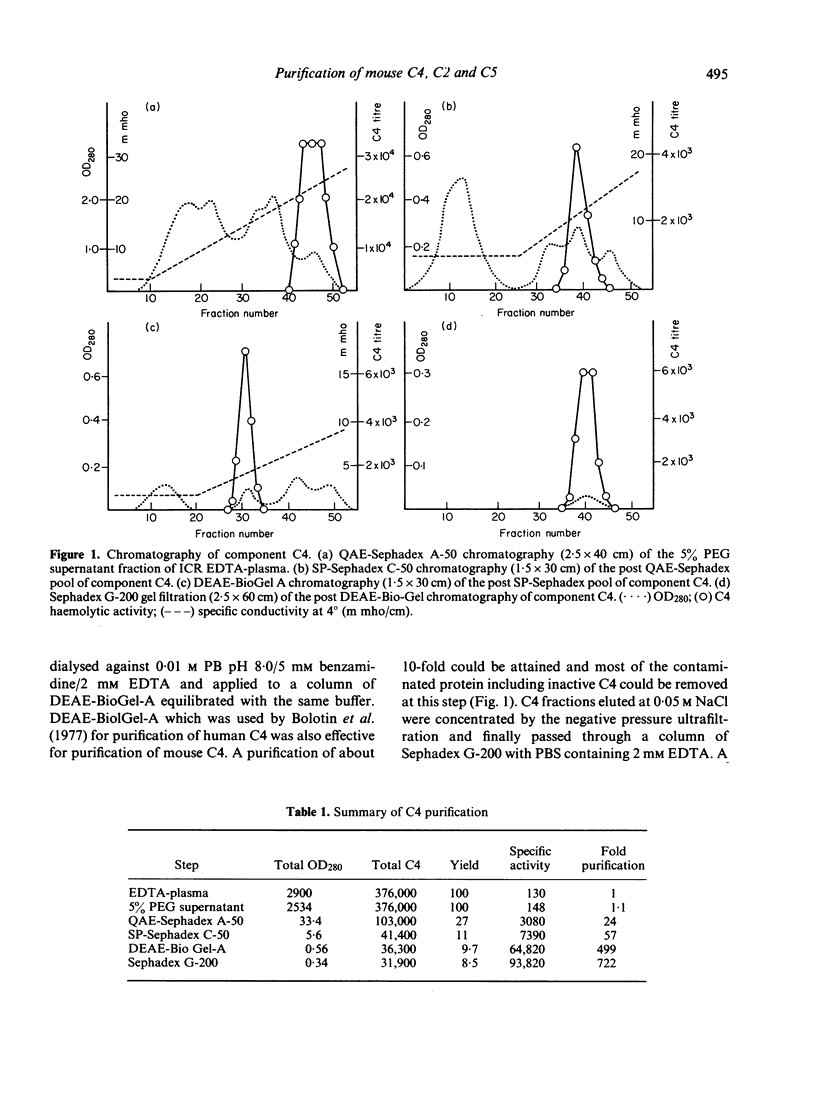
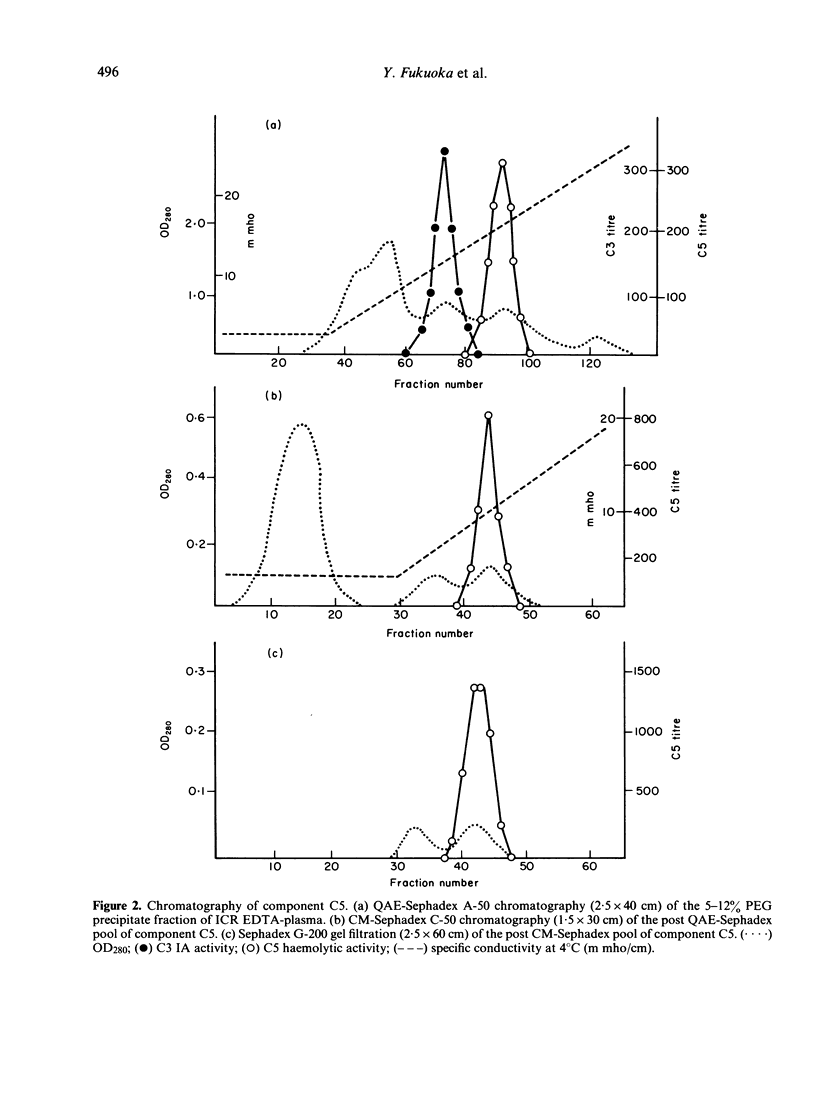
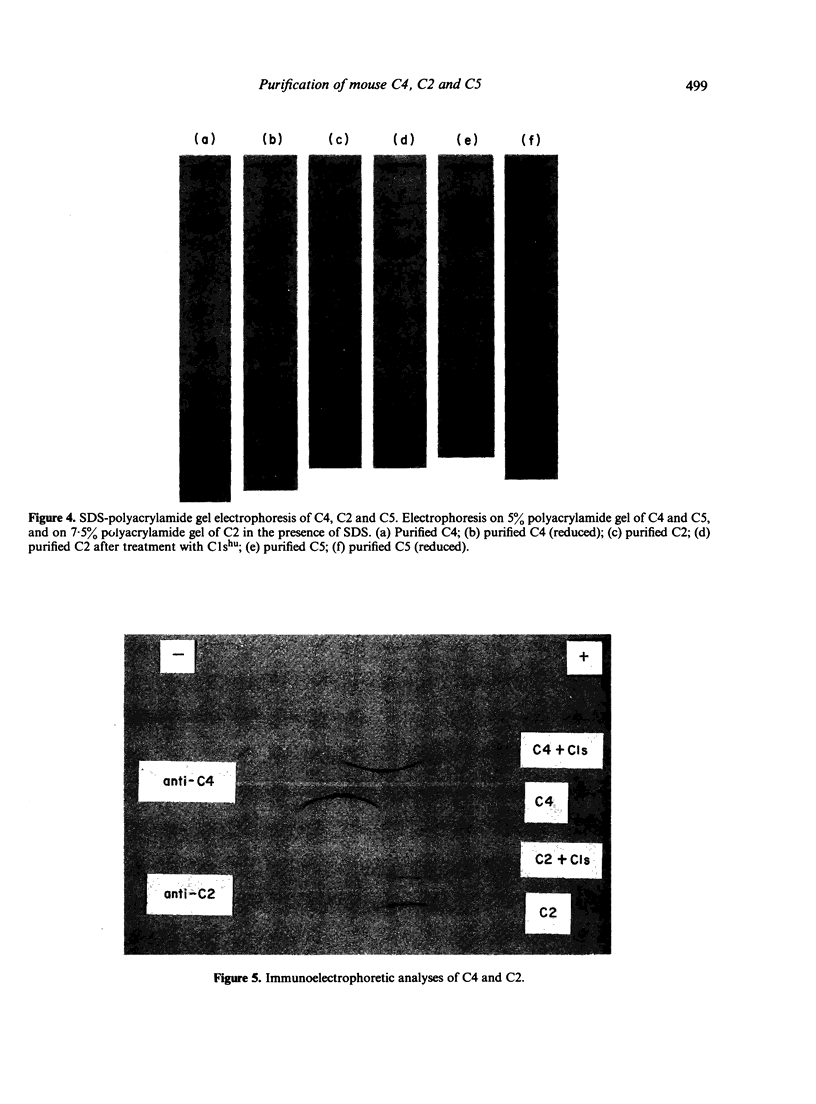
The fourth, second and fifth components of mouse complement were purified by a combination of polyethylene glycol precipitation, ion exchange chromatography and gel filtration. The final products were homogeneous on SDS-PAGE, and the activity yields were 8.5% for C4, 32% for C2 and 40% for C5. C4 was composed of three polypeptide chains with mol. wts of 90,000, 78,000 and 32,000. C2 was composed of a single polypeptide chain with a mol. wt. of 115,000 and cleaved by C1s into two fragments with mol. wts of 80,000 and 35,000. The half life of C4b2a was 7 min and was not prolonged by the iodination of C2. C2 activity could not be measured using EAC14hu or EAC14gp cells, but measurement was possible with the use of EAC14mo cells with purified C5 components of mouse complement. C5 was composed of two polypeptide chains with mol. wts of 135,000 and 84,000. This is the first report on the purification of functionally active mouse complement components C4, C2 and C5 from plasma.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P., McGinnis K., Shreffler D. Development and characterization of a hemolytic assay for mouse C4. J Immunol Methods. 1980;33(4):351–368. doi: 10.1016/0022-1759(80)90005-8. [DOI] [PubMed] [Google Scholar]
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Müller-Eberhard H. J. The reaction mechanism of human C5 in immune hemolysis. J Exp Med. 1970 Oct 1;132(4):775–793. doi: 10.1084/jem.132.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Nussenzweig V., Gigli I. Structural and functional differences between the H-2 controlled Ss and Slp proteins. J Exp Med. 1978 Nov 1;148(5):1186–1197. doi: 10.1084/jem.148.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka Y., Okuda T., Tachibana T. Variance in the activity of the fourth component of mouse complement. Tohoku J Exp Med. 1983 Oct;141(2):225–235. doi: 10.1620/tjem.141.225. [DOI] [PubMed] [Google Scholar]
- Fukuoka Y., Seino J., Okuda T., Tachibana T. Development of a hemolytic assay for mouse complement components in sera and the variation of their levels with age. Tohoku J Exp Med. 1982 May;137(1):79–90. doi: 10.1620/tjem.137.79. [DOI] [PubMed] [Google Scholar]
- Goldman M. B., Bangalore S., Goldman J. N. Functional and biochemical properties of the early classical complement system of mice. J Immunol. 1978 Jan;120(1):216–224. [PubMed] [Google Scholar]
- Gorman J. C., Jackson R., Desantola J. R., Shreffler D., Atkinson J. P. Development of a hemolytic assay for mouse C2 and determination of its genetic control. J Immunol. 1980 Jul;125(1):344–351. [PubMed] [Google Scholar]
- Kerr M. A., Gagnon J. The purification and properties of the second component of guinea-pig complement. Biochem J. 1982 Jul 1;205(1):59–67. doi: 10.1042/bj2050059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Hong K., Kondo K., Inoue K. Fifth component of guinea pig complement: purification and characterization. J Immunol. 1981 Jun;126(6):2414–2418. [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Cleavage of C2 by C1s into the antigenically distinct fragments C2a and C2b: demonstration of binding of C2b to C4b. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2998–3001. doi: 10.1073/pnas.74.7.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan S. H., Ward P. A. Generation of biologic activity from the purified alpha-chain of C5. J Immunol. 1979 Dec;123(6):2735–2740. [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos M. H., Kornfeld S., Shreffler D. C. Characterization of the oligosaccharide units of the fourth component of complement (Ss protein) synthesized by murine macrophages. J Immunol. 1980 Jun;124(6):2860–2863. [PubMed] [Google Scholar]
- SHREFFLER D. C. A SEROLOGICALLY DETECTED VARIANT IN MOUSE SERUM: FURTHER EVIDENCE FOR GENETIC CONTROL BY THE HISTOCOMPATIBILITY-2 LOCUS. Genetics. 1964 Jun;49:973–978. doi: 10.1093/genetics/49.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wetsel R. A., Kolb W. P. Complement-independent activation of the fifth component (C5) of human complement: limited trypsin digestion resulting in the expression of biological activity. J Immunol. 1982 May;128(5):2209–2216. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]