Abstract
Dantrolene is used for treatment of life-threatening hyperthermia, yet its thermoregulatory effects are unknown. We tested the hypothesis that dantrolene reduces the threshold (triggering core temperature) and gain (incremental increase) of shivering. With IRB approval and informed consent, healthy volunteers were evaluated on two random days: control and dantrolene (≈2.5 mg/kg plus a continuous infusion). In study 1, 9 men were warmed until sweating was provoked and then cooled until arterio-venous shunt constriction and shivering occurred. Sweating was quantified on the chest using a ventilated capsule. Absolute right middle fingertip blood flow was quantified using venous-occlusion volume plethysmography. A sustained increase in oxygen consumption identified the shivering threshold. In study 2, 9 men were given cold Ringer's solution IV to reduce core temperature ≈2°C/h. Cooling was stopped when shivering intensity no longer increased with further core cooling. The gain of shivering was the slope of oxygen consumption vs. core temperature regression. In Study 1, sweating and vasoconstriction thresholds were similar on both days. In contrast, shivering threshold decreased 0.3±0.3°C, P=0.004, on the dantrolene day. In Study 2, dantrolene decreased the shivering threshold from 36.7±0.2 to 36.3±0.3°C, P=0.01 and systemic gain from 353±144 to 211±93 ml·min−1·°C−1, P=0.02. Thus, dantrolene substantially decreased the gain of shivering, but produced little central thermoregulatory inhibition.
Keywords: Temperature: hyperthermia, fever; Pharmacology: dantrolene; Complications: shivering
Introduction
Fever results from a regulated increase in body temperature. It is mediated by an increase in circulating endogenous pyrogens that in turn increase the “setpoint” (1-3). Endogenous pyrogens cause a coordinated increase in the thermoregulatory threshold temperatures, causing the body to defend an abnormally high central temperature. The usual thermoregulatory effectors (vasoconstriction, nonshivering thermogenesis, shivering, and behavioral responses) are used to increase metabolic heat production and decrease heat loss to the environment. Fever associated with infection or allergy rarely exceeds 40°C.
In contrast, nonpyrogenic causes of hyperthermia are often serious, and sometimes life threatening. Grave causes include heat stroke (4,5), malignant hyperthermia (6), neuroleptic malignant syndrome (4), and drug-induced hyperthermia (7,8). Heat stroke results from extreme environmental temperatures, with or without concomitant exercise. Prevention of death or neurological sequelae from severe hyperthermia depends upon the prompt reduction of core temperature (7). The difficulty is that treatments for most types of extreme hyperthermia have been disappointing and there is no consensus that any given treatment is usually effective.
Dantrolene has been recognized as a specific treatment for malignant hyperthermia crises since 1975 (9). However, dantrolene is increasingly being used for emergency treatment of life-threatening hyperthermia that is unresponsive to conventional treatments. For example, the drug has been used with some success for acute treatment of life-threatening hyperthermia resulting from neuroleptic malignant syndrome (10-12) and hyperthermia associated with overdoses of various drugs. It has also been used for treatment of various other types of hyperthermia (12).
Efficacy in these cases appears to be based on a nonspecific action of the drug; but to the extent that dantrolene is effective, its action must conform to the laws of thermodynamics. Dantrolene must, therefore, reduce metabolic heat production, augment systemic heat loss, or alter the normal distribution of heat within the body. In other words, dantrolene must improve the abnormal (or ineffective) thermoregulatory control that initiates hyperthermic crises.
Dantrolene crosses the blood-brain barrier and produces mild sedation (13). Like other sedatives (14), it may thus have a central thermoregulatory action. However, the drug's primary mechanism of action is undoubtedly inhibition of excitation-contraction coupling skeletal muscles (15). It thus acts as a mild muscle relaxant — although of course by a different mechanism than conventional neuromuscular blockers. For example, dantrolene at a dose of 3.5 mg/kg substantially decreases twitch height in response to supramaximal electrical nerve stimulation in the pig (16). Muscle relaxation is of potential thermoregulatory importance because factors that reduce the efficacy of skeletal muscle activity will similarly reduce the efficacy of shivering. We therefore tested the hypothesis that dantrolene reduces the threshold (triggering core temperature) and gain (incremental response intensity) of shivering without influencing the sweating or vasoconstriction thresholds.
Methods
Eighteen healthy male volunteers participated in two protocols, each at a different institution. They were aged 18 to 45 yr and were ASA I. Exclusion criteria included any routine medications or a history of neuromuscular disease, Raynaud's syndrome, dysautonomia, or thyroid disease. Each participated in one of the two protocols described below.
Study 1
With approval of the IRB at Washington University and written informed consent, 9 volunteers participated on 2 different study days, each separated by a period of at least 3 days. The studies were timed so that major outcomes occurred at similar times to avoid circadian fluctuations in core temperature and the interthreshold range (17).
Volunteers had a light breakfast before arriving at the laboratory, but refrained from coffee and tea during the 8 hours before each investigation. During the study, they were allowed to drink juice and to eat crackers. The volunteers were minimally clothed and rested supine on a standard operating room table in a room maintained at ≈21°C with a relative humidity near 37%. A 14-g catheter was inserted in a right antecubital vein for blood sampling; an 18-g catheter was inserted in a left forearm vein for dantrolene or placebo administration.
The 2 study days were Dantrolene and Control. The treatment order was randomly assigned, but each participant was given each treatment. On the Dantrolene study day, volunteers were given 2.5 mg/kg of dantrolene at a rate of 5 mg·kg−1·h−1 for 30 minutes, followed by an infusion of 0.05 mg·kg−1·h−1. A comparable volume of saline was given on the placebo day.
One hour was allowed for dantrolene concentrations to reach steady-state values. Skin and core temperatures were then gradually increased with a forced-air cover and circulating-water mattress until significant sweating was observed (see Measurements, below). Skin and core temperatures were then gradually decreased, using the circulating-water mattress and a forced-air cooler. Temperature changes were restricted to <3°C/hour because this rate does not trigger dynamic thermoregulatory responses (18). The arms were protected from active warming and cooling throughout to avoid locally mediated vasomotion. However, all other skin below the neck was similarly manipulated. The study ended each day when shivering was detected. Before discharge, we confirmed adequate muscular strength by documenting that the volunteers were able to hold their heads off the bed for at least five seconds.
Heart rate was measured continuously using an electrocardiogram, and blood pressure was determined oscillometrically at 5-minute intervals. End-tidal PCO2 was measured with an Ultima monitor (Datex, Finland); exhaust gas from this monitor was returned to a DeltaTrac oxygen consumption monitor (Sensor Medics Corp., Yorba Linda, CA).
Core temperature was measured at the tympanic membrane using Mon-a-therm thermocouples (Tyco-Mallinckrodt Anesthesiology Products, Inc., St. Louis, MO). The aural probes were inserted by the volunteers until they felt the thermocouple touch the tympanic membrane; appropriate placement was confirmed when volunteers easily detected a gentle rubbing of the attached wire. The aural canal was occluded with cotton and taped in place. Mean skin temperature was calculated from 15 area-weighted measurements as previously described (19).
Skin and core thermocouples were connected to an Iso-Thermex (Columbus Instruments International Corp., Columbus, OH) 16-channel electronic thermometer with an accuracy of 0.1°C and a precision of 0.01°C. Core, and mean skin temperatures were recorded at 1-minute intervals.
Sweating was continuously quantified on the left upper chest using a ventilated capsule (18). We considered a sweating rate > 40 g·m−2·h−1 for at least 5 minutes to be significant (14). Absolute right middle fingertip blood flow was quantified using venous-occlusion volume plethysmography at 1-to-5-minute intervals (20). The vasoconstriction threshold was determined post hoc by an observer blinded to treatment and core temperature. As in previous similar studies (14), a DeltaTrac metabolic monitor (Yorba, CA) quantified shivering; the system was used in canopy-mode on both study days. This system provides measurements with an absolute accuracy of 5-10% (21-23). Measurements were averaged over one-minute intervals and recorded every minute.
Venous blood was sampled for dantrolene analysis at each threshold. Samples were centrifuged, and the plasma frozen at −30°C pending analysis. Plasma dantrolene concentrations were determined by reverse phase, high-performance liquid chromatography as previously described (24,25).
Heart rate, end-tidal PCO2, and oxyhemoglobin saturation (SpO2) were measured continuously using pulse oximetry, and blood pressure was determined oscillometrically at 5-min intervals at the left ankle.
Data analysis
The cutaneous contribution to sweating (26), vasoconstriction, and shivering is linear (27). We thus used measured skin and core temperatures in °C at each threshold to calculate the core-temperature threshold that would have been observed had skin been maintained at a single designated temperature:
| (Eq. 1) |
We have previously described the derivation and validation of this equation (14). We used a β of 0.1 for sweating (26) and a β of 0.2 for vasoconstriction and shivering (27). The designated skin temperature was set at 34°C, a typical intraoperative value.
Ambient temperature and humidity on each study day were first averaged within each volunteer; the resulting values were then averaged among volunteers.
Baseline values were recorded 15 minutes before thermal manipulations started. Calculated core-temperature thresholds for sweating, vasoconstriction, and shivering thresholds were determined in individual volunteers. Results on the Control and Dantrolene study days were compared with two-tailed, paired t tests. Data are presented as means ± SDs; P < 0.05 was considered statistically significant.
Study 2
With approval of the Human Studies Committee at the University of Louisville and written informed consent, 9 volunteers participated on 2 different study days, each separated by at least 3 days. The studies were timed so that major outcomes occurred at similar time of day to avoid circadian fluctuations in core temperature and the interthreshold range (17).
Each volunteer was evaluated on 2 study days, once without dantrolene (Control) and once with dantrolene (Dantrolene). On the Dantrolene study day, volunteers were give 2.5 mg/kg of dantrolene at a rate of 5 mg·kg−1·h−1 for 30 minutes, followed by an infusion of 0.05 mg.kg−1·h−1. A comparable volume of saline was given on the placebo day. Treatment order was randomized.
A 20-cm-long, 16-gauge catheter was inserted into a vein of the antecubital fossa for drug and fluid administration. A condom catheter prevented sympathetic nervous system activation that can result from the pain associated with a full bladder.
Throughout the study period, mean-skin temperature was kept near 31°C using a forced-air cover and circulating-water mattress. A mean-skin temperature of 31°C was used because it does not provoke shivering, but is low enough to allow a modest amount of cold fluid to trigger substantial shivering. Core cooling was started at least one hour after dantrolene administration to allow dantrolene plasma concentration to reach steady state. The core was cooled by administration of lactated Ringer's solution at ≈3°C (28). The cooling rate was restricted to ≤ 3°C/h because such rates are unlikely to trigger dynamic thermoregulatory responses (18). Cooling was stopped after 3-4 liters of cold fluid were given.
Heart rate, blood pressure and end-tidal PCO2 were measured as described above. Core and skin temperatures were similarly measured, as was oxygen consumption. Venous blood was sampled for dantrolene analysis at the beginning and end of the cold fluid infusion. Samples were centrifuged and the plasma frozen at −30°C pending analysis.
A sustained increase in oxygen consumption identified the shivering threshold. The gain of shivering was determined as the slope of oxygen consumption vs. core temperature regression during its ascent toward the maximum observed value. The data series was smoothed using a three-minute, running-average filter. The shivering threshold and gain were determined post hoc by an investigator blinded to treatment and core temperature.
Data Analysis
Hemodynamic responses, ambient temperature, relative humidity, and end-tidal PCO2 on each study day were averaged within each volunteer; data obtained between the onset of shivering and the maximum intensity or end of the cold fluid infusion were included. The resulting values were then averaged among volunteers. Results on the two study days were compared using two-tailed, paired t tests. The amount of fluid administered was non-parametrically distributed and thus compared using a Wilcoxon Signed-Rank test. Results are presented as means ± SDs; P < 0.05 was considered statistically significant.
Results
Study 1: Sweating, Vasoconstriction, and Shivering Thresholds
The participants were aged 25 ± 4 years, weighed 66 ± 11 kg, and were 172 ± 10 cm tall. Mean arterial pressure, heart rate, respiratory rate, ambient temperature, and relative humidity were comparable on the study days. Average end-tidal PCO2 was comparable on the Dantrolene and Control days, and lactated Ringer's solution requirement was similar (Table 1).
Table 1.
Confounding Factors and Major Outcomes for Study 1: Sweating, Vasoconstriction, and Shivering Thresholds.
| Control Day | Dantrolene Day | P value | |
|---|---|---|---|
| Mean Arterial Pressure (mmHg) | 79 ± 10 | 82 ± 6 | 0.23 |
| Heart Rate (bpm) | 65 ± 7 | 66 ± 8 | 0.34 |
| End Tidal PCO2(mmHg) | 39 ± 1 | 40 ± 2 | 0.20 |
| Fluids (L) | 0.8 ± 0.3 | 1.1 ± 0.5 | 0.11 |
| Ambient Temperature (°C) | 21.0 ± 2.0 | 21.3 ± 2.0 | 0.48 |
| Relative Humidity (%) | 37.2 ± 4.4 | 37.8 ± 5.3 | 0.80 |
Data are presented as means ± SDs; results were compared with paired t-tests.
The sweating threshold was similar on the control day and during dantrolene administration (37.2 ± 0.2 °C vs 37.1 ± 0.2 °C, P = 0.61) The vasoconstriction threshold was also similar on the control day and during dantrolene administration (36.7 ± 0.2°C. vs 36.7 ± 0.3°C, P = 0.93) In contrast, the shivering threshold decreased by 0.3 ± 0.3°C, P = 0.004 (Fig. 1; 35.8 ± 0.2°C for Control, 35.5 ± 0.4°C for Dantrolene). Dantrolene plasma concentrations were similar at each threshold (Table 2).
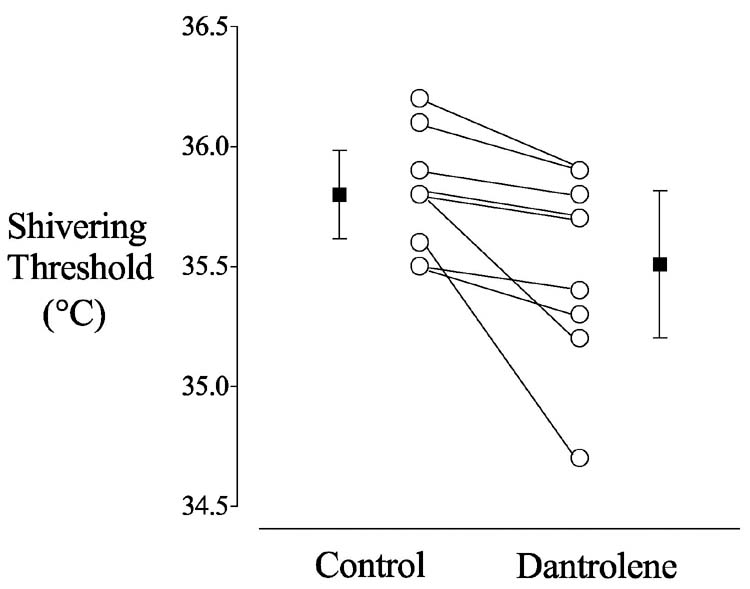
Fig. 1.
Shivering thresholds in nine volunteers who participated in Study 1 (Sweating, Vasoconstriction, and Shivering Thresholds). The open circles show the shivering threshold for each volunteer on the Control and Dantrolene days; the filled squares are the group means (± SDs). The shivering threshold was 0.3 ± 0.3°C greater on the Control day than on the Dantrolene day: 35.8 ± 0.2 vs. 35.5 ± 0.4°C, P = 0.004.
Table 2.
Mean Skin Temperatures, Core Temperatures, and Calculated Thresholds for Study 1: Sweating, Vasoconstriction, and Shivering Thresholds.
| Control | Dantrolene | ||
|---|---|---|---|
| Mean Skin (°C) | 36.6 ± 0.4 | 36.8 ± 0.5 | |
| Sweating | Core (°C) | 36.9 ± 0.2 | 36.9 ± 0.3 |
| Threshold (°C) | 37.2 ± 0.2 | 37.1 ± 0.2 | |
| [Dantrolene] (μg/ml) |
— |
5.2 ± 1.1 |
|
| Mean Skin (°C) | 33.4 ± 1.0 | 33.9 ± 1.2 | |
| Vasoconstriction | Core (°C) | 36.8 ± 0.3 | 36.7 ± 0.3 |
| Threshold (°C) | 36.7 ± 0.2 | 36.7 ± 0.3 | |
| [Dantrolene] (μg/ml) |
— |
5.5 ± 0.9 |
|
| Mean Skin (°C) | 29.2 ± 1.2 | 29.5 ± 1.3 | |
| Shivering | Core (°C) | 36.9 ± 0.3 | 36.6 ± 0.4 |
| Threshold (°C) | 35.8 ± 0.2 | 35.5 ± 0.4 | |
| [Dantrolene] (μg/ml) | — | 5.6 ± 1.0 |
Thresholds were calculated based on a designated mean-skin temperature of 34°C. Results are presented as means ± SDs. Only the thresholds were statistically compared, and only the shivering threshold differed significantly on the two treatment days (P=0.004).
Study 2: The Threshold and Gain of Shivering
The participants were aged 27 ± 7 years, weighed 72 ± 10 kg, and were 178 ± 10 cm tall. Mean arterial pressure, heart rate, respiratory rate, end-tidal PCO2, ambient temperature, and relative humidity were comparable on the study days. Significantly more lactated Ringer's solution was required on the Dantrolene day, but the difference was only 20% (Table 3).
Table 3.
Confounding Factors and Major Outcomes for Study 2: Threshold and Gain of Shivering.
| Control Day | Dantrolene Day | P value | |
|---|---|---|---|
| Mean Arterial Pressure (mmHg) | 109 ± 12 | 110 ± 13 | 0.87 |
| Heart Rate (bpm) | 73 ± 10 | 72 ± 12 | 0.32 |
| End Tidal PCO2 (mmHg) | 40 ± 4 | 41 ± 4 | 0.65 |
| Fluids (L) | 3.0 ± 0.5 | 3.6 ± 0.9 | 0.03* |
| Ambient Temperature (°C) | 22.8 ± 0.9 | 22.5 ± 1.3 | 0.29 |
| Relative Humidity (%) | 34.1 ± 6.7 | 33.4 ± 7.7 | 0.80 |
| Average Skin Temperature (°C) | 31.3 ± 0.4 | 31.3 ± 0.4 | 0.73 |
| Shivering Threshold (°C) | 36.7 ± 0.2 | 36.3 ± 0.3 | 0.01 |
| Gain of Shivering (ml·min−1·°C−1) | 353 ± 144 | 211 ± 93 | 0.02 |
Wilcoxon Signed-Rank test; all other data were analyzed with paired t-tests. Data presented as means ± SDs.
Mean skin temperature was near 31°C before and during cold fluid infusion on both study days. On the dantrolene study day, plasma drug concentrations averaged 7.5 ± 2.8 μg/ml.
Dantrolene significantly decreased the shivering threshold from 36.7 ± 0.2 vs. 36.3 ± 0.3°C, P = 0.01. Systemic gain of shivering, as determined by oxygen consumption, was reduced 39% from 353 ± 144 to 211 ± 93 ml·min−1·°C−1 (P = 0.02, Fig. 2). Individual correlation coefficients between oxygen consumption and core temperature averaged 0.78 ± 0.10 on the Control day and 0.68 ± 0.10 on the Dantrolene day.
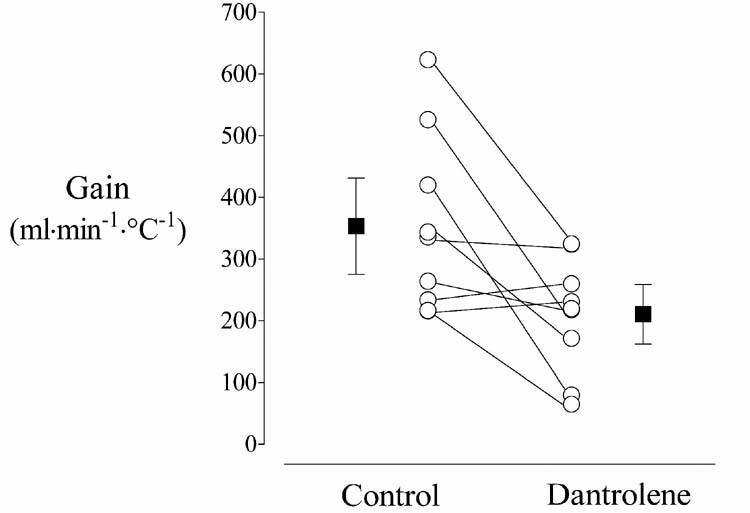
Fig. 2.
The gain of shivering in nine volunteers who participated in Study 2 (Threshold and Gain of Shivering). The open circles show the gain for each volunteer on the Control and Dantrolene days; the filled squares are the group means (± SDs). Gain was reduced 40% on the Dantrolene day: 353 ± 144 vs. 211 ± 93 ml·min−1·°C−1, P = 0.019.
Discussion
There are two mechanisms by which dantrolene might ameliorate hyperthermia. The first is central reduction in the vasoconstriction threshold, shivering threshold, or both. Dantrolene had no effect on vasoconstriction, but significantly reduced the shivering threshold. However, the reduction was only ≈0.3°C — an amount that compares poorly to other anti-shivering medications. For example, IV administration of 75 μg clonidine reduces the shivering threshold 0.6 ± 0.3°C (29) and this dose is sometimes effective for postoperative shivering (30), although larger doses were required in most studies (31). Furthermore, most anti-shivering drugs reduce the threshold considerably more (32). A reduction in the shivering threshold of only ≈0.3°C is thus unlikely to be of substantial clinical importance.
With the exceptions of meperidine (33) and nefopam (34), all drugs that centrally inhibit thermoregulatory control comparably reduce the vasoconstriction threshold. Since dantrolene had no effect on the vasoconstriction threshold, it seems likely that the small effect of dantrolene on the shivering was peripherally mediated.
The second mechanism by which dantrolene might ameliorate hyperthermia is a reduction in the gain of shivering. Here the effect of dantrolene was more impressive, with heat production increasing only 60% as much as without dantrolene. This magnitude is similar to the 50% reduction that results from administration of 0.7% isoflurane (35). Since sustained shivering roughly doubles basal metabolic rate (36), which is typically ≈70 kcal/h in a 70-kg adult (37), dantrolene administration might reduce shivering thermogenesis by as much as 30 kcal/h that would otherwise result from shivering. This corresponds to preventing an approximately 0.5°C/h increase in a core temperature (38). While not an enormous effect, this reduction is hardly clinically meaningless.
Although we cannot exclude a central action, the overwhelming likelihood is that dantrolene reduced the gain of shivering peripherally by reducing muscular strength. In other words, we have no reason to expect dantrolene to have a central action on nonspecific hyperthermia. A limitation of our study is that we were unable to determine maximum shivering intensity. Even so, given the peripheral relaxant effect of dantrolene, maximum shivering intensity is most likely reduced roughly in proportion to the reduction in gain.
A trivial effect on thermoregulatory response thresholds, combined with a substantial reduction in shivering gain, suggests that the action of dantrolene on nonspecific hyperthermia is restricted to relaxation of skeletal muscles. It would thus be logical to conclude that complete pharmacologic paralysis, which would obliterate shivering, would be even more effective than dantrolene administration. A corollary of this logic is that dantrolene is unlikely to be of any value in patients who are already paralyzed. At least in patients who are intubated, conventional muscle relaxants are safer, easier to use, and less expensive than dantrolene.
Dantrolene has been reported as being ineffective as a treatment for heat stroke (39); but in each case, it was given after physical activity had ceased and cooling measures started. It is not surprising that the drug failed under these conditions, because muscular activity was no longer contributing to hyperthermia and there is no specific failure of central thermoregulatory control during heat stroke. Instead, it results from a combination of excessive muscular activity and excessive environmental temperature.
The shivering threshold in the first study (Sweating, Vasoconstriction, and Shivering Thresholds) was less than in the second (Threshold and Gain of Shivering). This results largely from different methodologies. In the first, the threshold was calculated based on a designated skin temperature of 34°C; in contrast, skin temperature was controlled at 31°C in the second study. Skin temperature contributes 20% to control of shivering (27). Equation 1 thus indicates that we should expect the threshold for shivering to increase from 35.8°C to 36.5°C when skin temperature is reduced by 3°C. This is roughly what we observed and is consistent with previous observations in which the shivering threshold was determined by central infusion of cold fluid at a mean-skin temperature of 31°C (35).
A limitation of our protocol is that we tested only a single dose of dantrolene. The dose we used, ≈2.5 mg/kg, is standard for both pre-treatment of susceptible patients and treatment of acute malignant hyperthermia crises. It is a dose that is generally considered safe, although even small doses occasionally cause severe muscle weakness that can result in respiratory failure and mechanical ventilation (40). Larger doses would presumably have a greater effect. However, larger doses seem unlikely to alter our conclusions. Our results relate only to non-pharmacologically induced hyperthermia. Dantrolene's effects may be altered by the presence of pharmacologic agents (e.g., cocaine) that are known to be cause hyperthermia. In clinical practice, the effects of dantrolene will of course combine with the thermoregulatory effects of any other drugs that are being used. A natural consequence of reduced shivering gain is that the precise onset of shivering may be hard to detect. It is thus possible that the shivering threshold is reduced slightly less than 0.7°C. However, this would not change our conclusion that dantrolene only slightly impairs central control of shivering.
In summary, dantrolene substantially decreases the gain of shivering, but produces relatively little central thermoregulatory inhibition.
Footnotes
Received from the Outcomes Research™ Institute and Departments of Anesthesiology and Pharmacology, University of Louisville, Louisville, KY; the Department of Anesthesia, Washington University, St. Louis, MO; the Royal Melbourne Hospital, Melbourne, Victoria, Australia; and the Department of Anesthesia, University of Bern, Bern, Switzerland.
Presented in part at the 2002 annual meeting of the American Society of Anesthesiologists, Orlando, Florida, October 12-16.
Supported by National Institutes of Health Grants GM49670 and GM 061655 (Bethesda, MD), the Joseph Drown Foundation (Los Angeles, CA) and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). Dantrolene was a generous gift from Procter and Gamble (Cincinnati, OH). The authors do not consult for, accept honoraria from, or own stock or stock options in any company related to this research.
Implications statement: Dantrolene substantially decreases the gain of shivering, but produces relatively little central thermoregulatory inhibition. It thus seems unlikely to prove more effective than conventional muscle relaxants for treatment of life-threatening hyperthermia.
References
- 1.Nava F, Calapai G, Facciola G, et al. Effects of interleukin-10 on water intake, locomotory activity, and rectal temperature in rat treated with endotoxin. Int J Immunopharmacol. 1997;19:31–8. doi: 10.1016/s0192-0561(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz JI, Chan CC, Mukhopadhyay S, et al. Cyclooxygenase-2 inhibition by rofecoxib reverses naturally occurring fever in humans. Clin Pharmacol Ther. 1999;65:653–60. doi: 10.1016/S0009-9236(99)90087-5. [DOI] [PubMed] [Google Scholar]
- 3.Szelenyi Z, Bartho L, Szekely M, Romanovsky AA. Cholecystokinin octapeptide (CCK-8) injected into a cerebral ventricle induces a fever-like thermoregulatory response mediated by type B CCK- receptors in the rat. Brain Res. 1994;638:69–77. doi: 10.1016/0006-8993(94)90634-3. [DOI] [PubMed] [Google Scholar]
- 4.Ward A, Chaffman MO, Sorkin EM. Dantrolene. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs. 1986;32:130–68. doi: 10.2165/00003495-198632020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Moran D, Epstein Y, Wiener M, Horowitz M. Dantrolene and recovery from heat stroke. Aviat Space Environ Med. 1999;70:987–9. [PubMed] [Google Scholar]
- 6.Sessler DI. Malignant hyperthermia. J Pediatr. 1986;109:9–14. doi: 10.1016/s0022-3476(86)80563-7. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg J, Pentel P, Pond S, et al. Hyperthermia associated with drug intoxication. Crit Care Med. 1986;14:964–9. doi: 10.1097/00003246-198611000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Watson JD, Ferguson C, Hinds CJ, et al. Exertional heat stroke induced by amphetamine analogues: Does dantrolene have a place? Anaesthesia. 1993;48:1057–60. doi: 10.1111/j.1365-2044.1993.tb07526.x. [DOI] [PubMed] [Google Scholar]
- 9.Harrison G. Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium. Br J Anaesth. 1975;47 doi: 10.1093/bja/47.1.62. [DOI] [PubMed] [Google Scholar]
- 10.Goulon M, de Rohan-Chabot P, Elkharrat D, et al. Beneficial effects of dantrolene in the treatment of neuroleptic malignant syndrome: a report of two cases. Neurology. 1983;33:516–8. [PubMed] [Google Scholar]
- 11.Granato JE, Stern BJ, Ringel A, et al. Neuroleptic malignant syndrome: successful treatment with dantrolene and bromocriptine. Ann Neurol. 1983;14:89–90. doi: 10.1002/ana.410140117. [DOI] [PubMed] [Google Scholar]
- 12.Mann SC, Caroff SN, Peck PEJ. American Psychiatric publishing, Inc.; Washington, DC: 2003. Neuroleptic malignant syndrome and related conditions. [Google Scholar]
- 13.Meyler WJ, Bakker H, Kok JJ, et al. The effect of dantrolene sodium in relation to blood levels in spastic patients after prolonged administration. J Neurol Neurosurg Psychiatry. 1981;44:334–9. doi: 10.1136/jnnp.44.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsukawa T, Kurz A, Sessler DI, et al. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82:1169–80. doi: 10.1097/00000542-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Morgan KG, Bryant SH. The mechanism of action of dantrolene sodium. J Pharmacol Exp Ther. 1977;201:138–47. [PubMed] [Google Scholar]
- 16.Flewellen EH, Nelson TE. Dantrolene dose response in malignant hyperthermia-susceptible (MHS) swine: method to obtain prophylaxis and therapeusis. Anesthesiology. 1980;52:303–8. doi: 10.1097/00000542-198004000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Tayefeh F, Plattner O, Sessler DI, et al. Circadian changes in the sweating-tovasoconstriction interthreshold range. Pflügers Arch. 1998;435:402–6. doi: 10.1007/s004240050530. [DOI] [PubMed] [Google Scholar]
- 18.Lopez M, Sessler DI, Walter K, et al. Rate and gender dependence of the sweating, vasoconstriction, and shivering thresholds in humans. Anesthesiology. 1994;80:780–8. doi: 10.1097/00000542-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Sessler DI, Schroeder M. Heat loss in humans covered with cotton hospital blankets. Anesth Analg. 1993;77:73–7. doi: 10.1213/00000539-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Rubinstein EH, Sessler DI. Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology. 1990;73:541–5. [PubMed] [Google Scholar]
- 21.Tissot S, Delafosse B, Bertrand O, et al. Clinical validation of the Deltatrac monitoring system in mechanically ventilated patients. Int Care Med. 1995;21:149–53. doi: 10.1007/BF01726538. [DOI] [PubMed] [Google Scholar]
- 22.Weyland A, Weyland W, Sydow M, et al. Inverse fick's principle in comparison to measurements of oxygen consumption in respiratory gases. Does intrapulmonary oxygen uptake account for differences shown by different system methods? Anaesthesist. 1994;43:658–66. doi: 10.1007/s001010050106. [DOI] [PubMed] [Google Scholar]
- 23.Merilainen PT. Metabolic monitor. Int J Clin Monit Comput. 1987;4:167–77. doi: 10.1007/BF02915904. [DOI] [PubMed] [Google Scholar]
- 24.Lalande M, Mills P, Peterson RG. Determination of dantrolene and its reduced and oxidized metabolites in plasma by high-performance liquid chromatography. J Chromatogr. 1988;430:187–91. doi: 10.1016/s0378-4347(00)83151-5. [DOI] [PubMed] [Google Scholar]
- 25.Allen GC, Cattran CB, Peterson RG, Lalande M. Plasma levels of dantrolene following oral administration in malignant hyperthermia-susceptible patients. Anesthesiology. 1988;69:900–4. doi: 10.1097/00000542-198812000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Nadel ER, Horvath SM, Dawson CA, Tucker A. Sensitivity to central and peripheral thermal stimulation in man. J Appl Physiol. 1970;29:603–9. doi: 10.1152/jappl.1970.29.5.603. [DOI] [PubMed] [Google Scholar]
- 27.Cheng C, Matsukawa T, Sessler DI, et al. Increasing mean skin temperature linearly reduces the core-temperature thresholds for vasoconstriction and shivering in humans. Anesthesiology. 1995;82:1160–8. doi: 10.1097/00000542-199505000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Kurz A, Xiong J, Sessler DI, et al. Desflurane reduces the gain of thermoregulatory arterio-venous shunt vasoconstriction in humans. Anesthesiology. 1995;83:1212–9. doi: 10.1097/00000542-199512000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Delaunay L, Bonnet F, Liu N, et al. Clonidine comparably decreases the thermoregulatory thresholds for vasoconstriction and shivering in humans. Anesthesiology. 1993;79:470–4. doi: 10.1097/00000542-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Joris J, Banache M, Bonnet F, et al. Clonidine and ketanserin both are effective treatments for postanesthetic shivering. Anesthesiology. 1993;79:532–9. doi: 10.1097/00000542-199309000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Horn E-P, Werner C, Sessler DI, et al. Late intraoperative administration of clonidine prevents postanesthetic shiverirng after total intravenous or volatile anesthesia. Anesth Analg. 1997;84:613–7. doi: 10.1097/00000539-199703000-00028. [DOI] [PubMed] [Google Scholar]
- 32.Talke P, Tayefeh F, Sessler DI, et al. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87:835–41. doi: 10.1097/00000542-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Kurz A, Ikeda T, Sessler DI, et al. Meperidine decreases the shivering threshold twice as much as the vasoconstriction threshold. Anesthesiology. 1997;86:1046–54. doi: 10.1097/00000542-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Alfonsi P, Adam F, Sessler DI, et al. Nefopam inhibits thermoregulation and possesses a specific anti-shivering effect (abstract) Anesthesiology. 2002;97:A-257. [Google Scholar]
- 35.Ikeda T, Kim J-S, Sessler DI, et al. Isoflurane alters shivering patterns and reduces maximum shivering intensity. Anesthesiology. 1998;88:866–73. doi: 10.1097/00000542-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Horvath SM, Spurr GB, Hutt BK, Hamilton LH. Metabolic cost of shivering. J Appl Physiol. 1956;8:595–602. doi: 10.1152/jappl.1956.8.6.595. [DOI] [PubMed] [Google Scholar]
- 37.Matsukawa T, Sessler DI, Sessler AM, et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82:662–73. doi: 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Burton AC. Human calorimetry: The average temperature of the tissues of the body. J Nutr. 1935;9:261–80. [Google Scholar]
- 39.Bouchama A, Cafege A, Devol EB, et al. Ineffectiveness of dantrolene sodium in the treatment of heatstroke. Crit Care Med. 1991;19:176–80. doi: 10.1097/00003246-199102000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Watson CB, Reierson N, Norfleet EA. Clinically significant muscle weakness induced by oral dantrolene sodium prophylaxis for malignant hyperthermia. Anesthesiology. 1986;65:312–4. [PubMed] [Google Scholar]