Abstract
Alternative splicing is one of the central mechanisms that regulate eukaryotic gene expression. Here we report a tissue-specific RNA-binding protein, Fox-1, which regulates alternative splicing in vertebrates. Fox-1 bound specifically to a pentanucleotide GCAUG in vitro. In zebrafish and mouse, fox-1 is expressed in heart and skeletal muscles. As candidates for muscle-specific targets of Fox-1, we considered two genes, the human mitochondrial ATP synthase γ-subunit gene (F1γ) and the rat α-actinin gene, because their primary transcripts contain several copies of GCAUG. In transfection experiments, Fox-1 induced muscle-specific exon skipping of the F1γ gene via binding to GCAUG sequences upstream of the regulated exon. Fox-1 also regulated mutually exclusive splicing of the α-actinin gene, antagonizing the repressive effect of polypyrimidine tract-binding protein (PTB). It has been reported that GCAUG is essential for the alternative splicing regulation of several genes including fibronectin. We found that Fox-1 promoted inclusion of the fibronectin EIIIB exon. Thus, we conclude that Fox-1 plays key roles in both positive and negative regulation of tissue-specific splicing via GCAUG.
Keywords: alternative splicing/Fox-1/GCAUG/RNA binding
Introduction
Alternative splicing leads to the generation of functionally distinct proteins from a single gene and often controls how a gene acts during development and differentiation (reviewed in Lopez, 1998; Smith and Valcarcel, 2000; Grabowski and Black, 2001; Graveley, 2001; Maniatis and Tasic, 2002). The Drosophila sex determination pathway is the most striking example of regulation involving a cascade of regulated alternative splicing events (for reviews, see Inoue et al., 1995; Lopez, 1998; Smith and Valcarcel, 2000). For example, Sex-lethal (Sxl) protein induces female-specific splicing of transformer (tra) pre-mRNA, blocking the use of the non-sex-specific acceptor site, resulting in generation of functional Tra protein only in females. Tra and Tra-2 proteins bind to the exonic regulatory sequences and promote female-specific splicing of doublesex (dsx) pre-mRNA. In vertebrates, many genes are regulated by alternative splicing. It has been estimated that 40–60% of the human gene transcripts are alternatively spliced (for reviews, see Black, 2000; Graveley, 2001; Lander et al., 2001). Despite the importance of alternative splicing, many questions about how it is regulated remain unanswered.
Recently, a few tissue-specific regulators of alternative splicing have been reported in vertebrates. A neuron-specific RNA-binding protein, Nova-1, regulates alternative splicing in neurons: nova-1-null mice have a specific splicing defect in the inhibitory glycine receptor α2 (GlyRα2) exon 3A (Jensen et al., 2000). QKI-5, encoded by the mouse quaking gene, regulates alternative splicing of myelin-associated glycoprotein (MAG) (Wu et al., 2002). CELF/Bruno-like proteins play important roles in nervous-specific splicing and muscle-specific splicing (Ladd et al., 2001; Charlet et al., 2002; Suzuki et al., 2002; Zhang et al., 2002).
It has been shown that ubiquitously expressed factors, such as the SR proteins and the heterogeneous nuclear ribonucleoproteins (hnRNPs), play important roles in alternative splicing (reviewed in Smith and Valcarcel, 2000). Polypyrimidine tract-binding protein (PTB), or hnRNP I, regulates the alternative splicing of many genes such as c-src, FGF-R2, calcitonin/CGRP, GABA receptor γ2, α-tropomyosin and α-actinin (for reviews, see Smith and Valcarcel, 2000; Wagner and Garcia-Blanco, 2001). PTB functions as a negative regulator of alternative splicing, binding to intronic splicing silencer elements. It is likely that negative regulation of alternative splicing by PTB is derepressed by tissue- and stage-specific factors.
Other clues to the mechanisms of alternative splicing have emerged from studies of a splicing enhancer element, (U)GCAUG. The hexanucleotide UGCAUG has been found to be required for EIIIB exon inclusion of fibronectin (Huh and Hynes, 1994; Lim and Sharp, 1998). Moreover, UGCAUG and/or GCAUG are essential for exon inclusion in the splicing regulation of some genes such as c-src (Modafferi and Black, 1997), calcitonin/CGRP (Hedjran et al., 1997), non-muscle myosin heavy chain (NMHC)-B (Kawamoto, 1996) and 4.1R (Deguillien et al., 2001). A recent computational study on the human genome showed that UGCAUG often lies in flanking introns of brain-specific and muscle-specific exons (Brudno et al., 2001). Thus, it is highly likely that the GCAUG sequence plays key roles in tissue-specific splicing. However, nothing is known about possible regulatory protein(s) that bind to GCAUG.
In the present study, we report a tissue-specific RNA-binding protein, Fox-1, which binds specifically to the GCAUG sequence. In zebrafish, fox-1 is expressed during muscle development, whereas a mouse homologous gene is expressed in brain as well as heart and skeletal muscle. Here we show that Fox-1 promotes muscle-specific splicing via binding to GCAUG in transfection experiments. Our data suggest that Fox-1 plays key roles in both positive and negative regulation of tissue-specific splicing.
Results
fox-1 encodes a tissue-specific nuclear RNA-binding protein
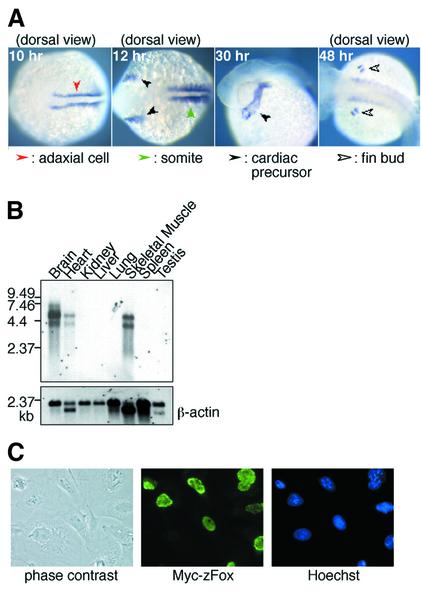
In Caenorhabditis elegans, fox-1 is involved in sex determination and dosage compensation, acting as a post-transcriptional repressor of the sex determination gene xol-1 (Meyer, 2000). It has been suggested that nematode fox-1 may regulate sex-specific splicing of xol-1 (Skipper et al., 1999). We therefore expected that a Fox-1-related protein might function as a splicing regulator in vertebrates. Here we identified an RNA-binding protein, zebrafish Fox-1 (zFox-1), that contains an RNA recognition motif (RRM) (Burd and Dreyfuss, 1994) and has homology to nematode Fox-1 (Hodgkin et al., 1994) and human ataxin2-binding protein A2BP1 (Shibata et al., 2000) (Figure 1). Whole-mount in situ hybridization analysis showed that zebrafish fox-1 was expressed specifically during muscle development: its mRNA was observed in adaxial cells, somites, cardiac precursors, fin buds and jaw muscle cells (Figure 2A; data not shown). Northern blotting analysis showed that mouse Fox-1/A2BP1 is expressed in heart, skeletal muscle and brain (Figure 2B), like human A2BP1 (Shibata et al., 2000). In addition, we found that both zebrafish and mouse Fox-1 proteins fused with a Myc tag were localized in the nucleus (Figure 2C; data not shown). These results suggest that Fox-1 is involved in nuclear RNA processing events in a tissue-specific manner.
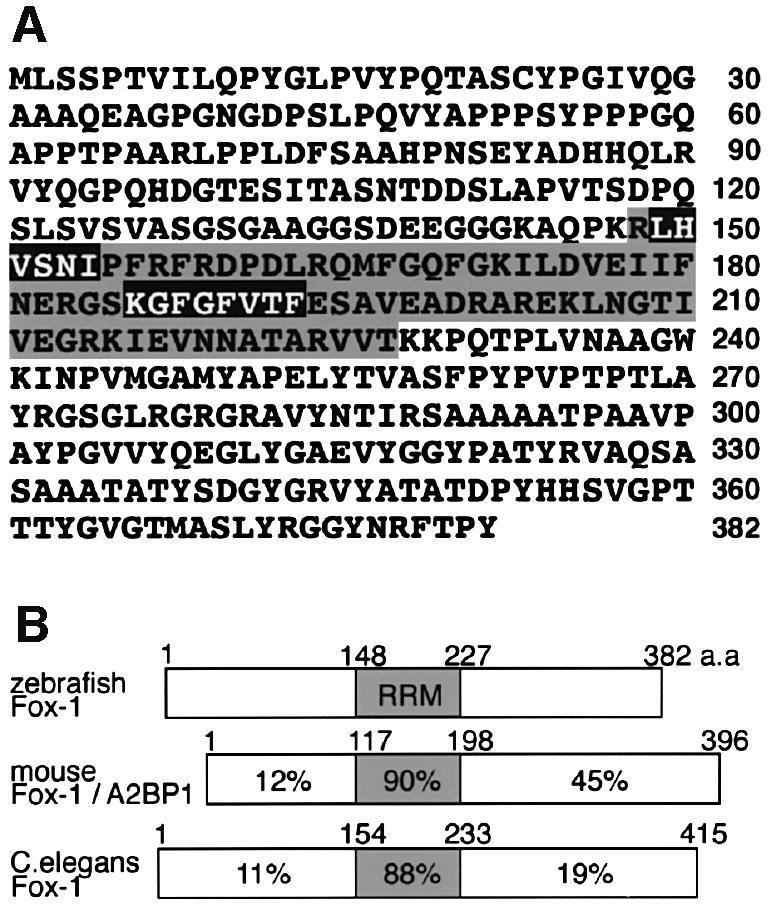
Fig. 1. Identification of zebrafish and mouse Fox-1 proteins. (A) Amino acid sequence of zebrafish Fox-1. The RNA recognition motif (RRM) is shaded (Burd and Dreyfuss, 1994). The RNP2 (hexamer) and RNP1 (octamer) motifs are boxed in black. (B) Schematic representation of zebrafish, mouse and nematode Fox-1 proteins. The percentage identity of amino acid sequences is shown.
Fig. 2. Expression of zebrafish and mouse fox-1 genes. (A) Whole-mount in situ hybridization of zebrafish embryos using a fox-1 probe. A dorsal view of a 10 h embryo shows fox-1 expression in adaxial cells, precursor cells of slow muscle. A dorsal view of a 12 h embryo shows fox-1 expression in somites and bilateral presumptive heart cells. Expression in the developing heart as well as in somites is observed at 30 h. A dorsal view of a 48 h embryo shows expression in the finbud cells. (B) Northern blot analysis of mouse Fox-1 (upper) and β-actin (lower) using MessageMap Blot (Stratagene). (C) Nuclear localization of Fox-1 protein fused with a Myc tag was detected in CV-1 cells. Hoechst staining of the cells is shown in the right panel.
Fox-1 binds to GCAUG in vitro
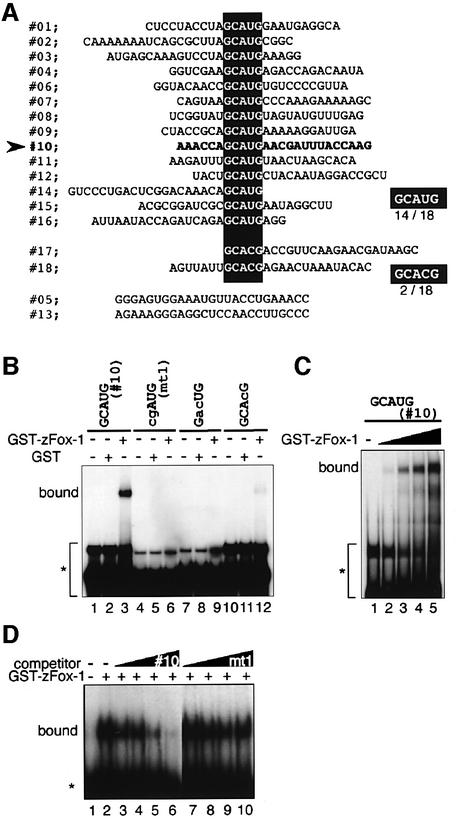
To identify target RNA molecules for Fox-1 protein, we performed an in vitro selection experiment using a zFox-1 protein fused with GST. After five rounds of selection and amplification, 18 cDNA clones were subjected to sequence analysis: 14 clones contained a GCAUG sequence and two clones a GCACG sequence (Figure 3A). The function of this sequence was examined by gel shift experiments (Figure 3B). When the No. 10 RNA probe, which contained the GCAUG sequence, was used, strong binding of zFox-1 was observed (lanes 1–3). Fox-1 bound to the GCACG sequence weakly (lanes 10–12). In contrast, zFox-1 binding was not detectable in the case of mutant RNA probes (lanes 4–9). Fox-1 binding was not detected in the case of the No. 13 probe, which contained neither GCAUG nor GCACG (data not shown). We found that binding efficiency to the GCAUG sequence was dependent on the amount of Fox-1 (Figure 3C). Moreover, binding of Fox-1 to GCAUG was competed out by GCAUG-containing RNA, not by RNA containing the mutant sequence (Figure 3D). These results clearly indicate that Fox-1 binds to GCAUG in vitro.
Fig. 3. Fox-1 protein binds specifically to GCAUG in vitro. (A) In the in vitro selection experiment, the sequences of 18 cDNA clones were aligned. The sequences GCAUG and GCACG are boxed. (B) Gel shift analyses of Fox-1 protein. No. 10 RNA (lanes 1–3) and mutant RNAs (lanes 4–12) were incubated with GST (lanes 2, 5, 8 and 11) or GST–zFox-1 (lanes 3, 6, 9 and 12). The position of unbound probe is shown by an asterisk. (C) Dose-dependent binding of Fox-1 protein to No. 10 RNA. Various amounts of GST–zFox-1 protein were incubated with the No. 10 RNA (0, 250, 500, 750 or 1000 ng, from left to right). (D) Competition experiments for GCAUG binding. No. 10 RNA was used as a probe. No.10 (lanes 3–6) or its mutant RNA (cgAUG) (lanes 7–10) was added as competitor (10-, 50-, 250-and 1250-fold excess compared with the 32P-labeled probe).
Fox-1 induces muscle-specific exon skipping of F1γ pre-mRNA via GCAUG
Our findings suggested that Fox-1 is involved in the regulation of muscle-specific splicing via GCAUG in vertebrates. Muscle-specific splicing has been well characterized for several mammalian genes, although there are no reports that GCAUG is involved in muscle-specific splicing. As candidates for muscle-specific tar gets of Fox-1, we focused on two genes, the human mitochondrial ATP synthase γ-subunit gene (F1γ) (Hayakawa et al., 2002) and the rat α-actinin gene (Southby et al., 1999), because their primary transcripts contain several copies of GCAUG.
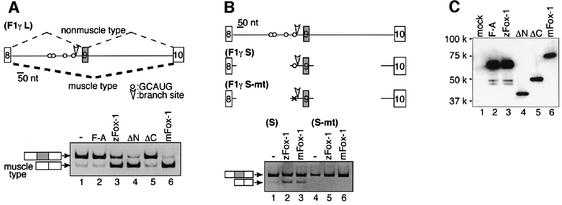
In the case of human F1γ, exon 9 is excluded from the splicing product in a muscle-specific manner (Hayakawa et al., 2002) (Figure 4A). We found several copies of the GCAUG sequence in intron 8 in the human and mouse F1γ genes. To examine whether Fox-1 controls alternative splicing of F1γ pre-mRNA, we performed transfection experiments with a human F1γ mini-gene. When the mini-gene hF1γL was transfected into mouse fibrosarcoma L929 cells, the predominant splicing product generated was non-muscle mRNA, and only a small amount of muscle-type product lacking exon 9 was detected (Figure 4A, lane 1). In contrast, muscle-type mRNA was produced efficiently in the presence of zebrafish or mouse Fox-1 (Figure 4A, lanes 3 and 6). Essentially the same results were obtained with a mouse F1γ mini-gene (Ichida et al., 2000) (data not shown). Furthermore, essentially the same results were obtained in mouse fibroblast NIH-3T3, monkey kidney epithelial CV-1 and mouse myoblast C2C12 cells (data not shown). Thus, Fox-1 can induce muscle-specific exon skipping of mammalian F1γ pre-mRNA irrespective of the cell type.
Fig. 4. Fox-1 induces muscle-specific splicing of the human mitochondrial ATP synthase F1γ gene via binding to GCAUG. (A) Transfection analyses with the hF1γL mini-gene. Exon 9 is skipped in a muscle-specific manner (Hayakawa et al., 2002). The positions of the branch site (arrowhead) and the GCAUG sequences (open circles) are shown in the schematic representation of the mini-gene. The mini-gene was transfected into L929 cells along with pCS2+ vector (Rupp et al., 1994) (lane 1), zFox-1 F190A (lane 2), zFox-1 (lane 3), zFox-1ΔN (lane 4), zFox-1ΔC (lane 5) or mouse Fox-1 (lane 6). The positions of non-muscle and muscle-type splicing products are indicated on the left. (B) Transfection analyses of the hF1γS mini-gene containing the Fox-1-binding sequence GCAUG (lanes 1–3) or the mutant sequence cgAUG (lanes 4–6) with pCS2+ MT vector (lanes 1 and 4), zFox-1 (lanes 2 and 5) or mouse Fox-1 (lanes 3 and 6). (C) Western blotting of the cell extracts to detect Fox-1 proteins: mock (lane 1), zFox-1 F190A (lane 2), zFox-1 (lane 3), zFox-1ΔN (lane 4), zFox-1ΔC (lane 5) and mouse Fox-1 (lane 6), expressed from the pCS2+ MT vector using the anti-Myc antibody.
To examine if the RNA-binding ability of Fox-1 is essential for the splicing regulation, we introduced an amino acid substitution into the RNP1 motif (Burd and Dreyfuss, 1994) of Fox-1. As expected, the mutant protein zFox-1 F190A did not have RNA-binding activity in vitro (data not shown). We confirmed the nuclear localization of zFox-1 F190A protein with a Myc tag (data not shown). When the hF1γL mini-gene was co-transfected with zFox-1 F190A, muscle-specific splicing was not induced (Figure 4A, lane 2), indicating that the RNA-binding activity of Fox-1 is indispensable for the splicing regulation.
We next examined whether Fox-1 regulates muscle-specific splicing via binding to GCAUG. We transfected an F1γ mini-gene (hF1γS) that contained only one copy of the GCAUG sequence in intron 8 due to a large deletion in the intronic sequence (Figure 4B). We found that exon 9 skipping was induced by zebrafish or mouse Fox-1 (Figure 4B, lanes 1–3). Next, we introduced base substitutions into the GCAUG sequence of hF1γS. Transfection analysis showed that the muscle-type mRNA from the resultant mini-gene hF1γS-mt was barely detectable even in the presence of Fox-1 (Figure 4B, lanes 4–6). These results indicate that Fox-1 induces muscle-specific splicing via binding to the GCAUG sequence in vertebrates.
In Drosophila, binding of Sxl protein to the non-sex-specific acceptor region of tra pre-mRNA is sufficient for induction of female-specific splicing (Inoue et al., 1990) via interference with an essential splicing factor, U2AF (Valcarcel et al., 1993). To examine whether Fox-1 induces muscle-specific exon skipping simply by binding to F1γ pre-mRNA, we expressed truncated zFox-1 proteins, ΔN and ΔC, fused with the SV40 nuclear localization signal (NLS). Both proteins were localized in the nucleus (data not shown). We found that zFox-1ΔC, in which the C-terminal 122 residues were truncated, did not induce muscle-specific splicing of the F1γ pre-mRNA, while zFox-1ΔN, in which the N-terminal 145 residues were truncated, induced muscle-specific splicing (Figure 4A, lanes 4 and 5). UV cross-linking experiments showed that the truncated Fox-1 proteins ΔN and ΔC had the same binding specificity as the intact Fox-1 protein in vitro (data not shown). These results suggest that the induction of muscle-specific exon skipping of F1γ pre-mRNA by Fox-1 probably involves the association of Fox-1 with some protein(s) via its C-terminal region.
Fox-1 regulates mutually exclusive splicing of α-actinin
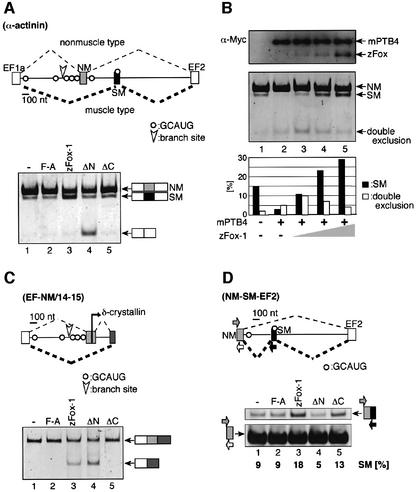
It has been reported that the rat α-actinin gene produces two mRNA isoforms in which the upstream non-muscle (NM) exon and the downstream smooth muscle (SM) exon are selected in a mutually exclusive manner (Southby et al., 1999) (Figure 5A). Several copies of the GCAUG sequence lie in the introns flanking the NM exon (Southby et al., 1999). When the actinin mini-gene (Southby et al., 1999) alone was transfected into CV-1 cells, mostly NM-type mRNA was generated, and only a small amount of SM mRNA was detected (Figure 5A, lane 1). When zFox-1 protein was co-expressed, the SM-type mRNA increased, with a concomitant decrease of the NM mRNA (lane 3). In contrast, zFox-1 F190A did not induce the SM-type splicing (lane 2). Essentially the same results were obtained in L929 cells (data not shown). These results indicate that zFox-1 induces SM-specific splicing of α-actinin.
Fig. 5. Muscle-specific splicing of the rat α-actinin gene is induced by Fox-1. (A) Transfection analyses of the rat α-actinin mini-gene (Southby et al., 1999; Suzuki et al., 2002). In the schematic representation, NM and SM represent non-muscle- and smooth muscle-specific exons, respectively. The branch site upstream of the NM exon and the GCAUG sequences are shown. The mini-gene was co-expressed in CV-1 cells along with pCS2+ MT vector (lane 1), zFox-1 F190A (lane 2), zFox-1 (lane 3), zFox-1ΔN (lane 4) or zFox-1ΔC (lane 5). The position of each mRNA product is indicated on the right. (B) Antagonism between the effects of Fox-1 and mouse PTB4. Transfection of the actinin mini-gene with pCS2+ MT vector (lane 1) or mouse PTB4 (lanes 2–5: 1.5 µg of the expression plasmid) and zFox-1 (lanes 3–5: 0.03, 0.09 and 0.27 µg, respectively, of the plasmid). The upper panel shows western blotting of the cell extracts to detect Fox-1 and PTB proteins expressed from the pCS2+ MT vector using the anti-Myc antibody. The splicing products from the actinin mini-gene were analyzed by RT–PCR (middle panel), and the amounts of SM mRNA (black bars) and EF1-EF2 mRNA (white bars) were expressed relative to the total amount of splicing products (lower panel). (C) Transfection analyses of the chimera construct, EF-NM/14–15, with pCS2+ MT vector (lane 1), zFox-1 F190A (lane 2), zFox-1 (lane 3), zFox-1ΔN (lane 4) or zFox-1ΔC (lane 5). The position of each mRNA product is shown schematically on the right. (D) Transfection analyses of the NM-SM-EF2 mini-gene with pCS2+ MT vector (lane 1), zFox-1 F190A (lane 2), zFox-1 (lane 3), zFox-1 ΔN (lane 4) or zFox-1 ΔC (lane 5). The primer sets are shown schematically (arrows). The fraction of SM exon inclusion (percentage) is shown at the bottom of each lane.
We considered three possibilities by which Fox-1 might regulate mutually exclusive splicing of α-actinin. The first possibility is that Fox-1 simply represses NM splicing, leading to the usage of the SM exon. The second possibility is that Fox-1 promotes the inclusion of the SM exon without repressing NM splicing. The third possibility is that Fox-1 not only represses NM splicing but also promotes SM splicing. To test whether Fox-1 represses NM splicing, we constructed a chimeric mini-gene, EF-NM/14–15, in which a fragment extending from the EF1a exon to the NM exon of the actinin gene was fused with a fragment extending from exon 14 to exon 15 of the chicken δ-crystallin gene (Figure 5C). The crystallin pre-mRNA containing exon 14 to exon 15 is known to be spliced constitutively (Ohno et al., 1987). Transfection experiments showed that an mRNA product containing the chimeric exon NM/14 was generated exclusively in the absence of Fox-1 (Figure 5C, lane 1). In contrast, chimera exon skipping was induced by Fox-1 (lane 3). zFox-1 F190A mutant protein did not induce exon skipping (lane 2). These results indicate that NM splicing is repressed by Fox-1. Furthermore, we found that zFox- 1ΔC did not induce exon skipping, although zFox-1ΔN did, suggesting that the C-terminal region of zFox-1 plays a role in repressing NM splicing (lanes 4 and 5). Using the original actinin mini-gene, we further examined the effects of truncated Fox-1 proteins (Figure 5A, lanes 4 and 5). When zFox-1ΔC was expressed, SM splicing was not induced (lane 5). Unexpectedly, when zFox-1ΔN was expressed, muscle-specific splicing of actinin pre-mRNA was not induced. Instead, an additional mRNA product lacking both the NM and SM exons was produced (lane 4). We designated this process ‘double exclusion’. It is likely that zFox-1ΔN can induce NM exon skipping, but not SM exon inclusion, resulting in double exclusion. The results suggested that Fox-1 promotes SM exon inclusion. To test this possibility further, we constructed an actinin mini-gene extending from the NM exon to the EF2 exon (NM-SM-EF2). When cells were transfected with the mini-gene alone, exclusion of the SM exon was mainly observed (Figure 5D, lane 1). In contrast, SM exon inclusion was enhanced by Fox-1, although the enhancement was not so efficient (lane 3). zFox-1 F190A did not induce SM exon inclusion (lane 2). Furthermore, zFox-1ΔN did not induce SM exon inclusion, although zFox-1ΔC did (lanes 4 and 5), which was consistent with the results shown in Figure 5A. Taking these results together, we conclude that Fox-1 not only represses NM splicing but also activates SM splicing of rat α-actinin.
It has been reported that PTB plays a key role in exclusion of the SM exon of actinin pre-mRNA in non-muscle cells (Southby et al., 1999; Wollerton et al., 2001). We found that double exclusion was slightly induced by mouse PTB4, with concomitant loss of the SM mRNA (Figure 5B, lanes 1 and 2). In the presence of mouse PTB4, a small amount of Fox-1 induced double exclusion as well as SM splicing (lane 3), suggesting that both the NM and SM exons are repressed in a fraction of actinin pre-mRNA. However, a larger amount of Fox-1 promoted SM splicing, with a concomitant decrease of double exclusion (lanes 4 and 5). These results indicate that Fox-1 antagonizes the repressive effect of PTB to promote muscle-specific splicing of α-actinin.
Fox-1 promotes inclusion of fibronectin EIIIB exon
It is known that the GCAUG sequence plays a pivotal role in the splicing regulation of some genes. In those cases, the GCAUG sequence is essential for exon inclusion. We examined whether Fox-1 regulates the inclusion of the fibronectin EIIIB exon (Huh and Hynes, 1994; Lim and Sharp, 1998). When the fibronectin 7iBi89 mini-gene (Huh and Hynes, 1993, 1994) alone was transfected into CV-1 cells, EIIIB exon inclusion was barely detectable (Figure 6, lane 1). Co-transfection of a plasmid expressing zebrafish or mouse Fox-1 induced a low but significant amount of EIIIB exon inclusion (lanes 3 and 6). The mutant Fox-1 protein F190A did not induce the exon inclusion (lane 2). These results clearly indicated that Fox-1 can induce the inclusion of the fibronectin EIIIB exon. In addition, the truncated Fox-1 proteins ΔN and ΔC did not induce the EIIIB exon inclusion (lanes 4 and 5), suggesting that both the N- and C-terminal portions of Fox-1 protein are important for promoting exon inclusion.
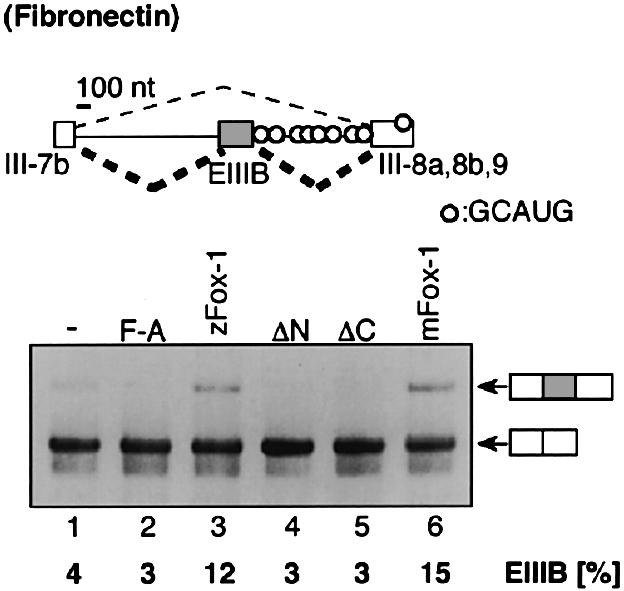
Fig. 6. Inclusion of the rat fibronectin EIIIB exon is promoted by Fox-1. The fibronectin 7iBi89 mini-gene (Huh and Hynes, 1993, 1994) was transfected into CV-1 cells with pCS2+ MT vector (lane 1), zFox-1 F190A (lane 2), zFox-1 (lane 3), zFox-1ΔN (lane 4), zFox-1ΔC (lane 5) or mouse Fox-1 (lane 6). The position of each mRNA product is shown on the right. The fraction of EIIIB exon inclusion (percentage) is shown at the bottom of each lane.
Discussion
Here we showed that Fox-1 regulated muscle-specific splicing of the F1γ and α-actinin genes as well as alternative splicing of the fibronectin EIIIB exon. We demonstrated that Fox-1 binding to GCAUG is essential for splicing regulation, at least in the case of F1γ pre-mRNA. It is also noteworthy that Fox-1 functions as both a negative and positive regulator of alternative splicing.
We found that mouse Fox-1 (Figure 2B) and human A2BP1 (Shibata et al., 2000) are expressed in the brain as well as in the heart and muscles. In mammals, neuron-specific splicing of NMHC-B and c-src is regulated by the GCAUG sequence (Kawamoto, 1996; Modafferi and Black, 1997). It has been shown that the hexanucleotide UGCAUG often lies in flanking introns of brain-specific exons and muscle-specific exons (Brudno et al., 2001). Therefore, we think it possible that Fox-1 is involved in brain-specific splicing as well as muscle-specific splicing via GCAUG.
Our present data strongly suggest that Fox-1 protein interacts with some other protein(s). Human A2BP1 was identified originally as a protein interacting with ataxin-2 protein in yeast two-hybrid screening: the C-terminal fragment of human A2BP1 is required for strong interaction with ataxin-2 (Shibata et al., 2000). The present study showed that the N-terminal portion of zFox-1 is required for positive regulation of splicing, while the C-terminal region is essential for both positive and negative regulation. Thus, it is likely that Fox-1 forms complexes with different proteins to exert these differing activities.
We demonstrated here that Fox-1 induces exon skipping via GCAUG in F1γ pre-mRNA: base substitution in this element in F1γ pre-mRNA resulted in disruption of exon skipping dependent on Fox-1 (Figure 4). This is the first report showing that the GCAUG sequence functions as a splicing silencer. In the case of F1γS pre-mRNA that contained only a single copy of the element, production of muscle-specific mRNA was not so efficient. When three copies of GCAUG were inserted into F1γS-mt, the efficiency of muscle-specific splicing was restored almost to the original level, further supporting the notion that GCAUG functions as a splicing silencer (our unpublished observations).
In the case of α-actinin pre-mRNA, Fox-1 not only repressed NM splicing but also promoted SM splicing (Figure 5). Our data showed that Fox-1 antagonizes the repressive effect of PTB to promote SM splicing, although whether Fox-1 interacts with PTB has not been examined. A recent study showed that one of the CELF/Bruno-like protein family members, Etr-3, induces muscle-specific splicing of cardiac troponin T (cTnT) pre-mRNA, antagonizing the repressive effect of PTB (Charlet et al., 2002). In addition, a brain-specific PTB (brPTB) interacts with Nova-1 and antagonizes the ability of Nova-1 to regulate neuron-specific splicing (Polydorides et al., 2000). Thus, antagonism between PTB and tissue-specific regulators plays a pivotal role in alternative splicing.
Our previous study revealed that zebrafish CELF/Bruno-like proteins, Bruno-like (Brul) and Etr-3, can induce muscle-specific splicing of rat α-actinin via binding to uridine and purine repeat elements (UREs) at the branch point upstream of the NM exon (Suzuki et al., 2002). Co-expression of Fox-1 and Brul resulted in the increased induction of muscle-specific splicing of actinin pre-mRNA, not in a synergistic, but rather in an additive, manner (our unpublished observations). Base substitutions in the URE sequence disrupted the regulation of muscle-specific splicing by Brul, while no significant effect was observed in the case of muscle-specific splicing induced by Fox-1 (our unpublished observations). These results suggest that CELF/Bruno-like proteins and Fox-1 function independently of each other, although it remains possible that CELF/Bruno-like proteins as well as Fox-1 promote muscle-specific splicing of actinin pre-mRNA through some PTB-antagonistic effect.
Fox-1 weakly promoted fibronectin EIIIB exon inclusion (Figure 6). Alternative splicing of the EIIIB exon is regulated in various tissues and stages (Huh and Hynes, 1994; Lim and Sharp, 1998). Another mouse fox-1-related gene, fxh (or RBM9), has been identified as a gene induced by androgens in motor neuron cells (Lieberman et al., 2001). Its mRNA is expressed in various tissues such as heart, brain, lung, liver and kidney (Lieberman et al., 2001). We found that mouse Fxh/RBM9 protein promotes fibronectin EIIIB exon inclusion, somewhat more strongly than Fox-1, suggesting its role in splicing regulation in various tissues (unpublished observations). We think that there are at least two possibilities for how Fox-1 (or a Fox-1-related protein) promotes fibronectin EIIIB exon inclusion. The first possibility is that Fox-1 directly induces assembly of splicing factors through binding to the GCAUG sequences. The second possibility is that Fox-1 antagonizes the repressive effect of PTB, as is the case for regulation of SM splicing in actinin pre-mRNA. It has been reported that PTB is involved in the regulation of fibronectin EIIIB exon inclusion (Norton and Hynes, 1993; Norton, 1994).
In C.elegans, it has been suggested that fox-1 regulates sex-specific splicing of xol-1 pre-mRNA (Skipper et al., 1999). Exon 7 is skipped in hermaphrodites, i.e. in the presence of Fox-1 (Meyer, 2000). We found two copies of GCAUG in the intron upstream of exon 7, and several copies of GCACG in the flanking introns. Furthermore, the nematode Fox-1 protein was able to induce muscle-specific exon skipping of the F1γ pre-mRNA in mammalian cells (our unpublished observations). Thus, it is possible that the mechanism of regulation of alternative splicing by Fox-1 proteins is somehow conserved in widely divergent species. We expect that further studies of Fox-1 will provide novel and significant information about the regulation of alternative splicing.
Materials and methods
Plasmids
A zebrafish neurula cDNA library in the ZAPII vector was screened to obtain a fox-1 cDNA clone (DDBJ/EMBL/GenBank accession No. AB074763). The mouse PTB4 cDNA was cloned by RT–PCR using total RNA from E9.5 whole embryos (DDBJ/EMBL/GenBank accession No. AB074764). The coding sequences of zebrafish Fox-1, mouse Fox-1/A2BP1 (NM_021477) and mouse PTB4 were cloned into pBluescript SK (Stratagene), pGEX-6P-1 (Pharmacia) and pCS2+ MT (Rupp et al., 1994) vectors. To construct deletion mutants of Fox-1 (ΔC and ΔN), the fox-1 cDNA fragments corresponding to nucleotides 1–780 and 463–1146, respectively, were inserted into a pCS2+ MT-derived vector that contained the sequence coding for the SV40 NLS (PKKKRKVKL). Mutagenesis was carried out using a Quikchange site-directed mutagenesis kit (Stratagene). The hF1γL mini-gene includes genomic fragments corresponding to nucleotides 13 648–13 853 and 17 433–18 568 of the human F1γ gene (Matsuda et al., 1993) in the pcDEB vector (Ichida et al., 2000), while the hF1γS mini-gene (pF1γEx8-9-10; Hayakawa et al., 2002) includes genomic fragments corresponding to nucleotides 13 650–13 741, 17 733–17 866 and 18 405–18 572 in the pCMV-SPORT vector (Life Technologies Inc.). A chimeric mini-gene, EF-NM/14–15, was constructed as follows. The genomic fragment of the α-actinin gene corresponding to nucleotides 51–1151 (Southby et al., 1999) was amplified by PCR and cloned into pCS2+ (Rupp et al., 1994) (pEF-NM). The HindIII–XbaI fragment of pSP14–15 (Sawa et al., 1988) was inserted into pEF-NM. To construct the NM-SM-EF2 mini-gene, the genomic fragment of the α-actinin gene corresponding to nucleotides 1132–2923 (Southby et al., 1999) was amplified by PCR and cloned into pCS2+ (Rupp et al., 1994).
In situ hybridization and northern blotting
Whole-mount in situ hybridization of zebrafish embryos was performed essentially as described previously (Maegawa et al., 1999). The RNA probe was prepared from fox-1 cDNA in pBluescript SK (Stratagene) using T3 RNA polymerase. Northern blot analysis of mouse Fox-1 was performed using MessageMap Northern Blot (Stratagene) as described previously (Maegawa et al., 1999).
SELEX and RNA-binding experiments
Recombinant GST fusion proteins were induced in Escherichia coli BL21(DE3) or DH5, and purified on glutathione–agarose beads (Pharmacia). In vitro selection experiments were carried out essentially as described previously (Abe et al., 1996; Suzuki et al., 2002). The starting RNA source was synthesized in vitro from a mixture of DNA templates containing a T7 promoter and a randomized 25 nucleotide region. A total of five rounds of selection and amplification were performed. The final cDNA products were inserted into pSP65 (Promega). Preparation of RNA probes and in vitro binding experiments were performed essentially as described previously (Ohno et al., 1990; Suzuki et al., 2002).
Transfection experiments
L929 and CV-1 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Transfection was performed by the calcium phosphate DNA precipitation method as described previously (Suzuki et al., 2002). The Myc fusion proteins expressed in transformant cells were examined by western blotting and immunofluorescence detection using anti-Myc antibody (cMyc 9E10; Santa Cruz Biotechnology). To analyze splicing products, RT–PCR was performed using the following oligonucleotides. For hF1γ(L), F1-2903 (GTCATCACAAAAGAGTTGATTG) and F1-2389 (CACTGCATTC TAGTTGTGGTTTGT); for hF1γ (S), F1-2903 and the T7 primer (Stratagene); and for fibronectin, 7iBi89-S (TTCGAATTCATC AGAGTTCCTGCACT) and hGHpolyA-AS (CTGCTCGAGACTGG AGTGGCAACTTC) were used. For NM-SM-EF2, NM-S (CGG CTCGAGGATCACTCCGGCACGTTGGG) and SM-AS (CCACTC GAGAACCCATGGAGATAAGGCAG) were used to analyze SM exon inclusion, while NM-S and NM-AS (GGGTCGTTGCCAATATC) were used to detect total splicing products. For α-actinin except NM-SM-EF2, ACTs121 and ACTas2873 were used (Suzuki et al., 2002). The RT–PCR products were electrophoresed in 5% native polyacrylamide gels or 2% agarose gels. After staining with Sybr green I (Molecular Probe), the products were analyzed using an FM-BIO II bioimager (Hitachi). Each transfection experiment was performed more than three times to confirm the reproducibility.
Acknowledgments
Acknowledgements
We are grateful to Dr Adrian Krainer for critical comments on the manuscript, Dr David J.Grunwald for the zebrafish cDNA library, Drs Justine Southby and Christopher W.J.Smith for the actinin mini-gene, Dr Richard O.Hynes for the fibronectin mini-gene, and Haruki Ochi for helpful discussions. This work was supported by Grant-in-Aids for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and a JSPS Research Fellowships for Young Scientists.
References
- Abe R., Sakashita,E., Yamamoto,K. and Sakamoto,H. (1996) Two different RNA binding activities for the AU-rich element and the poly(A) sequence of the mouse neuronal protein mHuC. Nucleic Acids Res., 24, 4895–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D.L. (2000) Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell, 103, 367–370. [DOI] [PubMed] [Google Scholar]
- Brudno M., Gelfand,M.S., Spengler,S., Zorn,M., Dubchak,I. and Conboy,J.G. (2001) Computational analysis of candidate intron regulatory elements for tissue-specific alternative pre-mRNA splicing. Nucleic Acids Res., 29, 2338–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C.G. and Dreyfuss,G. (1994) Conserved structures and diversity of functions of RNA-binding proteins. Science, 265, 615–621. [DOI] [PubMed] [Google Scholar]
- Charlet B.N., Logan,P., Singh,G. and Cooper,T.A. (2002) Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol. Cell, 9, 649–658. [DOI] [PubMed] [Google Scholar]
- Deguillien M., Huang,S.C., Moriniere,M., Dreumont,N., Benz,E.J.,Jr and Baklouti,F. (2001) Multiple cis elements regulate an alternative splicing event at 4.1R pre-mRNA during erythroid differentiation. Blood, 98, 3809–3816. [DOI] [PubMed] [Google Scholar]
- Grabowski P.J. and Black,D.L. (2001) Alternative RNA splicing in the nervous system. Prog. Neurobiol., 65, 289–308. [DOI] [PubMed] [Google Scholar]
- Graveley B.R. (2001) Alternative splicing: increasing diversity in the proteomic world. Trends Genet., 17, 100–107. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Sakashita,E., Ueno,E., Tominaga,S., Hamamoto,T., Kagawa,Y. and Endo,H. (2002) Muscle-specific exonic splicing silencer for exon exclusion in human ATP synthase γ-subunit pre-mRNA. J. Biol. Chem., 277, 6974–6984. [DOI] [PubMed] [Google Scholar]
- Hedjran F., Yeakley,J.M., Huh,G.S., Hynes,R.O. and Rosenfeld,M.G. (1997) Control of alternative pre-mRNA splicing by distributed pentameric repeats. Proc. Natl Acad. Sci. USA, 94, 12343–12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., Zellan,J.D. and Albertson,D.G. (1994) Identification of a candidate primary sex determination locus, fox-1, on the X chromosome of Caenorhabditis elegans. Development, 120, 3681–3689. [DOI] [PubMed] [Google Scholar]
- Huh G.S. and Hynes,R.O. (1993) Elements regulating an alternatively spliced exon of the rat fibronectin gene. Mol. Cell. Biol., 13, 5301–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh G.S. and Hynes,R.O. (1994) Regulation of alternative pre-mRNA splicing by a novel repeated hexanucleotide element. Genes Dev., 8, 1561–1574. [DOI] [PubMed] [Google Scholar]
- Ichida M., Hakamata,Y., Hayakawa,M., Ueno,E., Ikeda,U., Shimada,K., Hamamoto,T., Kagawa,Y. and Endo,H. (2000) Differential regulation of exonic regulatory elements for muscle-specific alternative splicing during myogenesis and cardiogenesis. J. Biol. Chem., 275, 15992–16001. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hoshijima,K., Sakamoto,H. and Shimura,Y. (1990) Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature, 344, 461–463. [DOI] [PubMed] [Google Scholar]
- Inoue K., Ohno,M. and Shimura,Y. (1995) Aspects of splice site selection in constitutive and alternative pre-mRNA splicing. Gene Expr., 4, 177–182. [PMC free article] [PubMed] [Google Scholar]
- Jensen K.B., Dredge,B.K., Stefani,G., Zhong,R., Buckanovich,R.J., Okano,H.J., Yang,Y.Y. and Darnell,R.B. (2000) Nova-1 regulates neuron-specific alternative splicing and is essential for neuronal viability. Neuron, 25, 359–371. [DOI] [PubMed] [Google Scholar]
- Kawamoto S. (1996) Neuron-specific alternative splicing of nonmuscle myosin II heavy chain-B pre-mRNA requires a cis-acting intron sequence. J. Biol. Chem., 271, 17613–17616. [PubMed] [Google Scholar]
- Ladd A.N., Charlet,B.N.N. and Cooper,T.A. (2001) The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol. Cell. Biol., 21, 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E.S. et al. (2001) Initial sequencing and analysis of the human genome. Nature, 409, 860–921. [DOI] [PubMed] [Google Scholar]
- Lieberman A.P., Friedlich,D.L., Harmison,G., Howell,B.W., Jordan, C.L., Breedlove,S.M. and Fischbeck,K.H. (2001) Androgens regulate the mammalian homologues of invertebrate sex determination genes tra-2 and fox-1. Biochem. Biophys. Res. Commun., 282, 499–506. [DOI] [PubMed] [Google Scholar]
- Lim L.P. and Sharp,P.A. (1998) Alternative splicing of the fibronectin EIIIB exon depends on specific TGCATG repeats. Mol. Cell. Biol., 18, 3900–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A.J. (1998) Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu. Rev. Genet., 32, 279–305. [DOI] [PubMed] [Google Scholar]
- Maegawa S., Yasuda,K. and Inoue,K. (1999) Maternal mRNA localization of zebrafish DAZ-like gene. Mech. Dev., 81, 223–226. [DOI] [PubMed] [Google Scholar]
- Maniatis T. and Tasic,B. (2002) Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature, 418, 236–243. [DOI] [PubMed] [Google Scholar]
- Matsuda C., Endo,H., Ohta,S. and Kagawa,Y. (1993) Gene structure of human mitochondrial ATP synthase γ-subunit. Tissue specificity produced by alternative RNA splicing. J. Biol. Chem., 268, 24950–24958. [PubMed] [Google Scholar]
- Meyer B.J. (2000) Sex in the wormcounting and compensating X-chromosome dose. Trends Genet., 16, 247–253. [DOI] [PubMed] [Google Scholar]
- Modafferi E.F. and Black,D.L. (1997) A complex intronic splicing enhancer from the c-src pre-mRNA activates inclusion of a heterologous exon. Mol. Cell. Biol., 17, 6537–6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P.A. (1994) Polypyrimidine tract sequences direct selection of alternative branch sites and influence protein binding. Nucleic Acids Res., 22, 3854–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P.A. and Hynes,R.O. (1993) Characterization of HeLa nuclear factors which interact with a conditionally processed rat fibronectin pre-mRNA. Biochem. Biophys. Res. Commun., 195, 215–221. [DOI] [PubMed] [Google Scholar]
- Ohno M., Sakamoto,H. and Shimura,Y. (1987) Preferential excision of the 5′ proximal intron from mRNA precursors with two introns as mediated by the cap structure. Proc. Natl Acad. Sci. USA, 84, 5187–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M., Kataoka,N. and Shimura,Y. (1990) A nuclear cap binding protein from HeLa cells. Nucleic Acids Res., 18, 6989–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polydorides A.D., Okano,H.J., Yang,Y.Y., Stefani,G. and Darnell,R.B. (2000) A brain-enriched polypyrimidine tract-binding protein antagonizes the ability of Nova to regulate neuron-specific alternative splicing. Proc. Natl Acad. Sci. USA, 97, 6350–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R.A., Snider,L. and Weintraub,H. (1994) Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev., 8, 1311–1323. [DOI] [PubMed] [Google Scholar]
- Sawa H., Ohno,M., Sakamoto,H. and Shimura,Y. (1988) Requirement of ATP in the second step of the pre-mRNA splicing reaction. Nucleic Acids Res., 16, 3157–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H., Huynh,D.P. and Pulst,S.M. (2000) A novel protein with RNA-binding motifs interacts with ataxin-2. Hum. Mol. Genet., 9, 1303–1313. [DOI] [PubMed] [Google Scholar]
- Skipper M., Milne,C.A. and Hodgkin,J. (1999) Genetic and molecular analysis of fox-1, a numerator element involved in Caenorhabditis elegans primary sex determination. Genetics, 151, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.W. and Valcarcel,J. (2000) Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem. Sci., 25, 381–388. [DOI] [PubMed] [Google Scholar]
- Southby J., Gooding,C. and Smith,C.W. (1999) Polypyrimidine tract binding protein functions as a repressor to regulate alternative splicing of α-actinin mutally exclusive exons. Mol. Cell. Biol., 19, 2699–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Jin,Y., Otani,H., Yasuda,K. and Inoue,K. (2002) Regulation of alternative splicing of α-actinin transcript by Bruno-like proteins. Genes Cells, 7, 133–141. [DOI] [PubMed] [Google Scholar]
- Valcarcel J., Singh,R., Zamore,P.D. and Green,M.R. (1993) The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature, 362, 171–175. [DOI] [PubMed] [Google Scholar]
- Wagner E.J. and Garcia-Blanco,M.A. (2001) Polypyrimidine tract binding protein antagonizes exon definition. Mol. Cell. Biol., 21, 3281–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollerton M.C., Gooding,C., Robinson,F., Brown,E.C., Jackson,R.J. and Smith,C.W. (2001) Differential alternative splicing activity of isoforms of polypyrimidine tract binding protein (PTB). RNA, 7, 819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.I., Reed,R.B., Grabowski,P.J. and Artzt,K. (2002) Function of quaking in myelination: regulation of alternative splicing. Proc. Natl Acad. Sci. USA, 99, 4233–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Liu,H., Han,K. and Grabowski,P.J. (2002) Region-specific alternative splicing in the nervous system: implications for regulation by the RNA-binding protein NAPOR. RNA, 8, 671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]