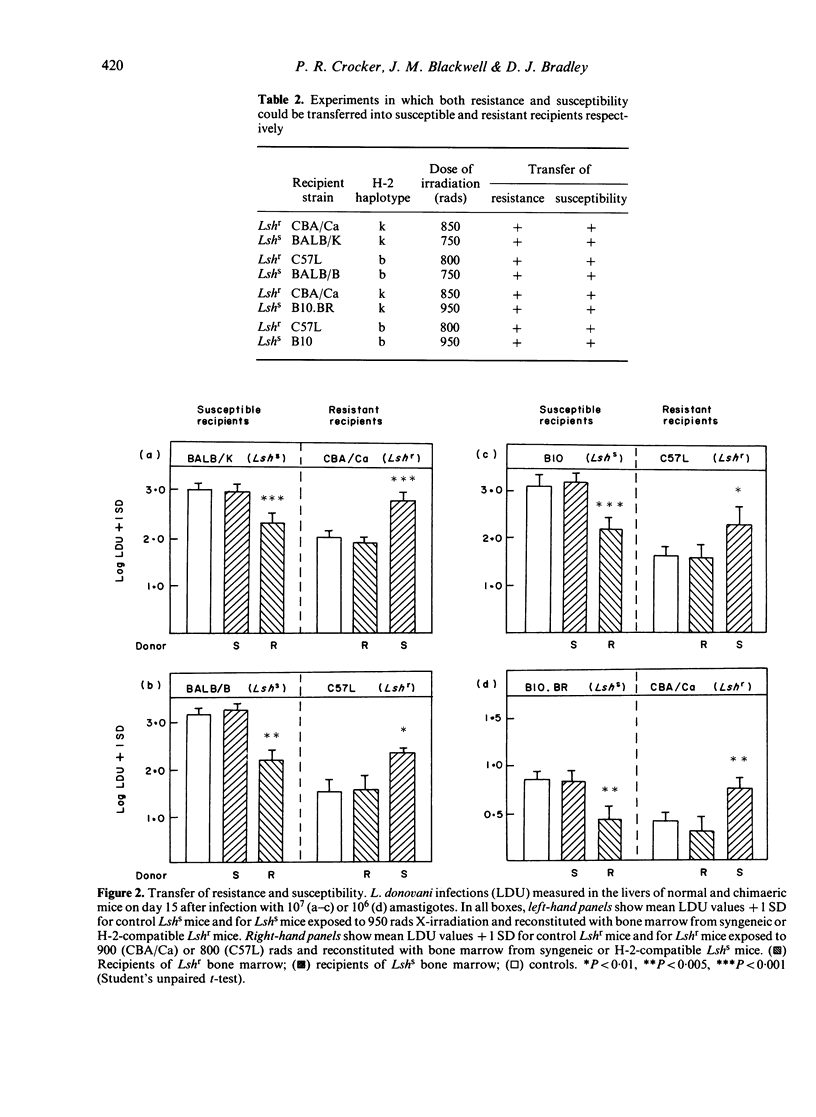
Abstract
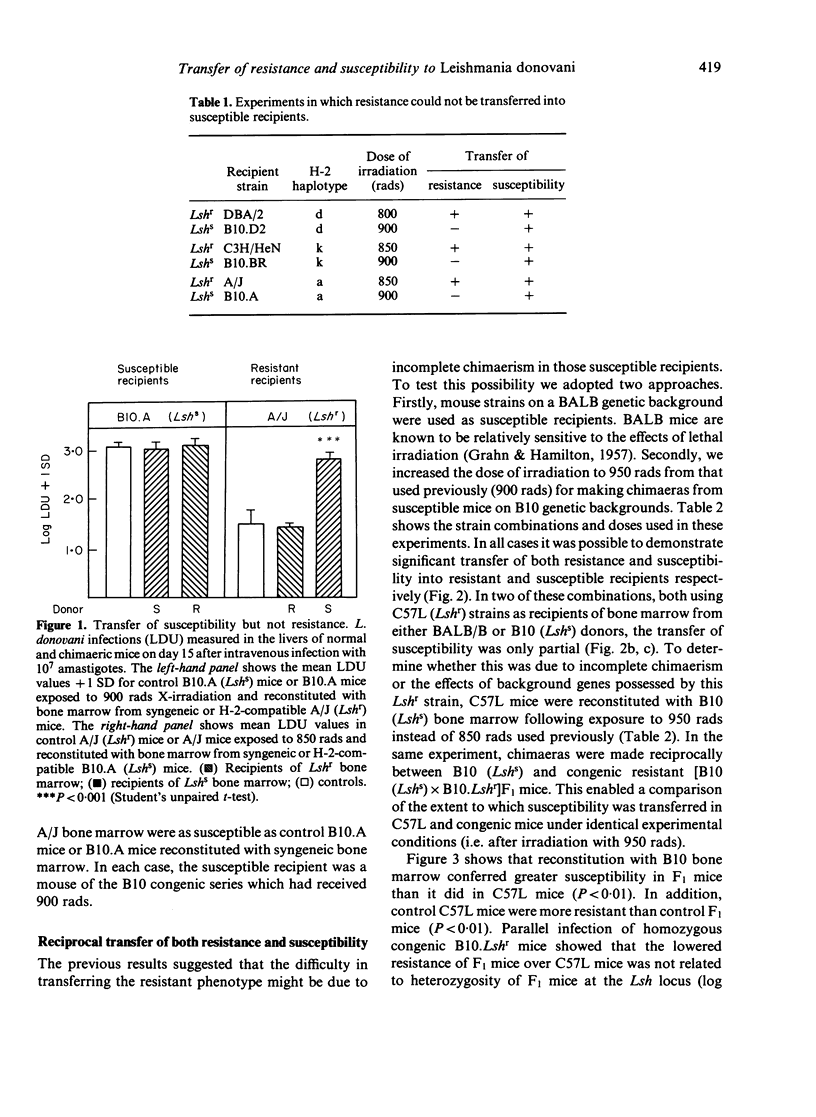
Reciprocal radiation bone marrow chimaeras were made between H-2-compatible strains of mice innately resistant or susceptible to visceral leishmaniasis. In initial experiments, susceptibility but not resistance to Leishmania donovani could be transferred with donor bone marrow into irradiated recipients. In subsequent experiments it was possible to transfer both resistance and susceptibility. This was achieved either by selecting more radiosensitive mouse strains as susceptible recipients, or alternatively by increasing the irradiation dose for the susceptible recipients used in the initial experiments. Using the higher irradiation dose, successful transfer of resistance and susceptibility between congenic mice carrying the Lshr and Lshs alleles on the more radioresistant B10 genetic background provided firm evidence that the results obtained in this study were specifically related to expression of the Lsh gene. We conclude that Lsh gene-controlled resistance and susceptibility to L. donovani is determined by bone marrow-derived cells. The cell type(s) involved is likely to be of the macrophage lineage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. J., Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977 Oct;30(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Variation in susceptibility of mouse strains to Leishmania donovani infection. Trans R Soc Trop Med Hyg. 1972;66(4):527–528. [PubMed] [Google Scholar]
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Blackwell J. M., Bradley D. J. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984 Mar;43(3):1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D, Hamilton K F. Genetic Variation in the Acute Lethal Response of Four Inbred Mouse Strains to Whole Body X-Irradiation. Genetics. 1957 May;42(3):189–198. doi: 10.1093/genetics/42.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E., Maskell D. J., Harrington K., Joysey H., Brock J. Mechanisms of natural resistance to mouse typhoid. Bull Eur Physiopathol Respir. 1983 Mar-Apr;19(2):137–142. [PubMed] [Google Scholar]
- Hormaeche C. E. The natural resistance of radiation chimeras to S. typhimurium C5. Immunology. 1979 Jun;37(2):329–332. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Genetically determined susceptibility to Leishmania tropica infection is expressed by haematopoietic donor cells in mouse radiation chimaeras. Nature. 1980 Nov 13;288(5787):161–162. doi: 10.1038/288161a0. [DOI] [PubMed] [Google Scholar]
- Kongshavn P. A., Sadarangani C., Skamene E. Genetically determined differences in antibacterial activity of macrophages are expressed in the environment in which the macrophage precursors mature. Cell Immunol. 1980 Aug 1;53(2):341–349. doi: 10.1016/0008-8749(80)90334-2. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J Immunol. 1982 Oct;129(4):1349–1351. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Formal S. B. Effect of silica on the innate resistance of inbred mice to Salmonella typhimurium infection. Infect Immun. 1979 Aug;25(2):513–520. doi: 10.1128/iai.25.2.513-520.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J. E., Blackwell J. M., O'Brien A. D., Bradley D. J., Glynn A. A. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature. 1982 Jun 10;297(5866):510–511. doi: 10.1038/297510a0. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Weinblatt A. C., O'Brien A. D. Genetic control of resistance to infection in mice. Crit Rev Immunol. 1982 Jun;3(4):263–330. [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Ulczak O. M., Blackwell J. M. Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: the effects of parasite dose, cyclophosphamide and sublethal irradiation. Parasite Immunol. 1983 Sep;5(5):449–463. doi: 10.1111/j.1365-3024.1983.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Virolainen M. Hematopoietic origin of macrophages as studied by chromosome markers in mice. J Exp Med. 1968 May 1;127(5):943–952. doi: 10.1084/jem.127.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]


