Abstract
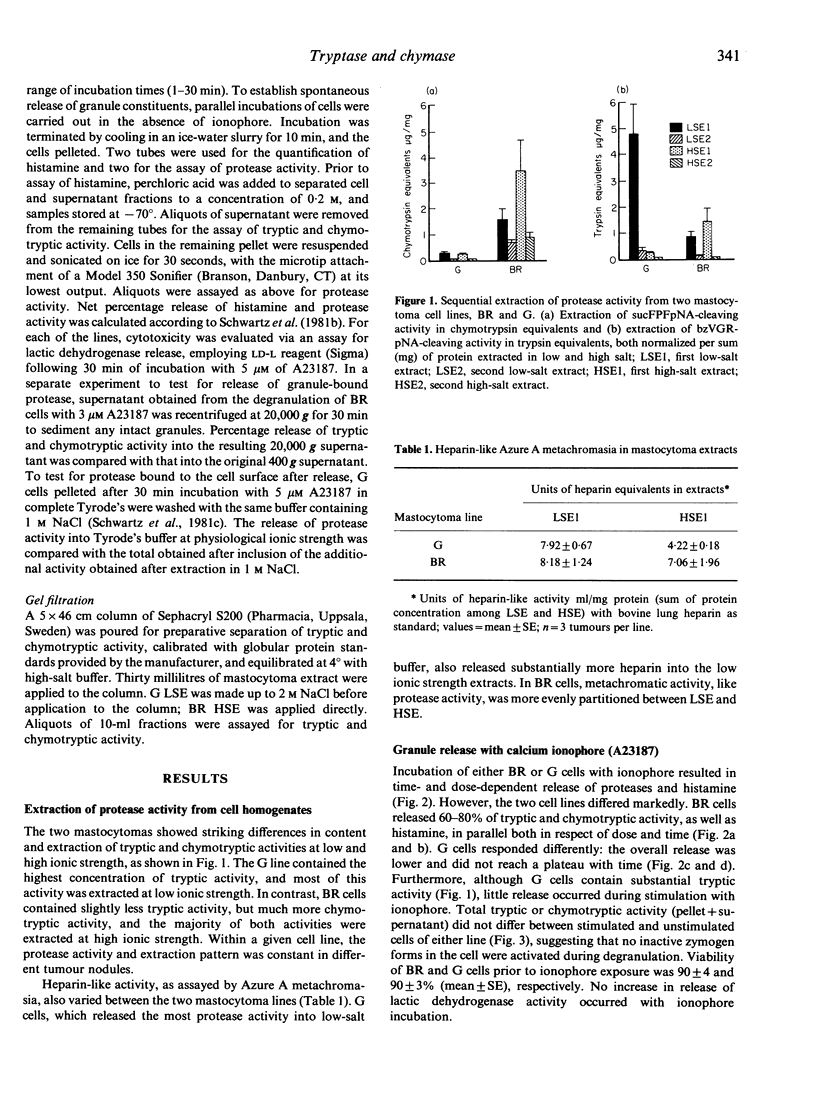
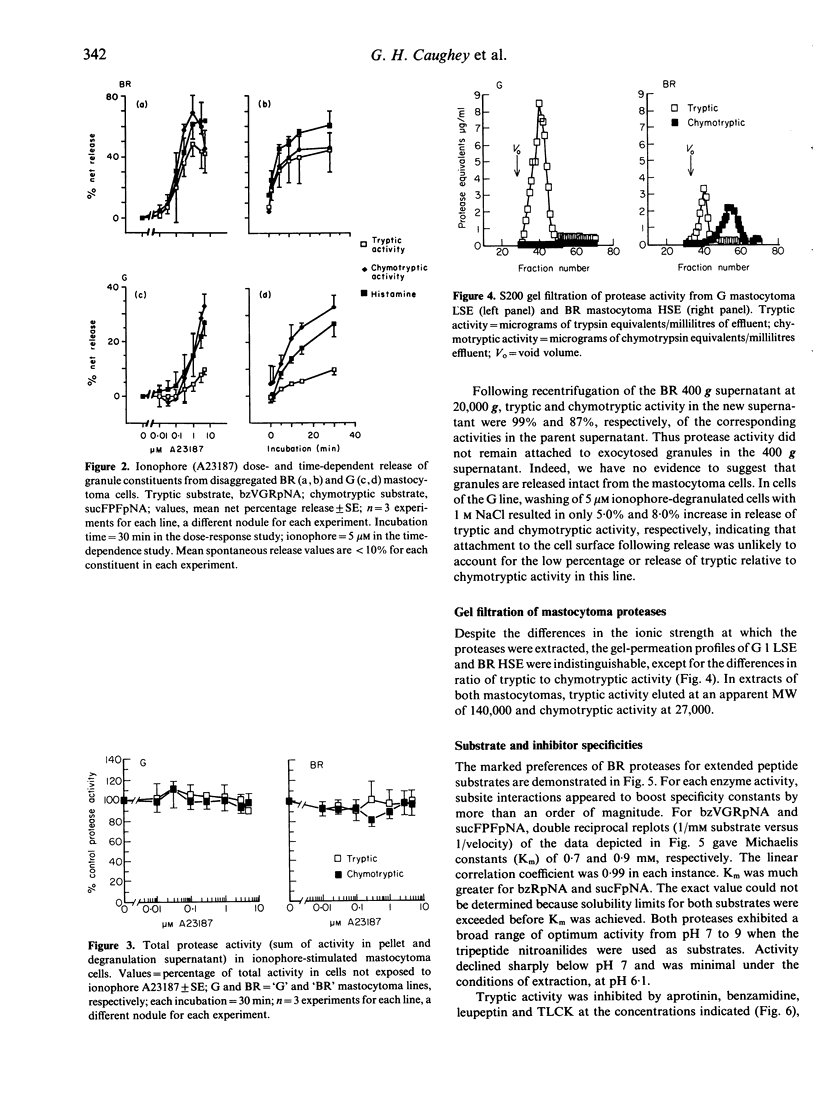
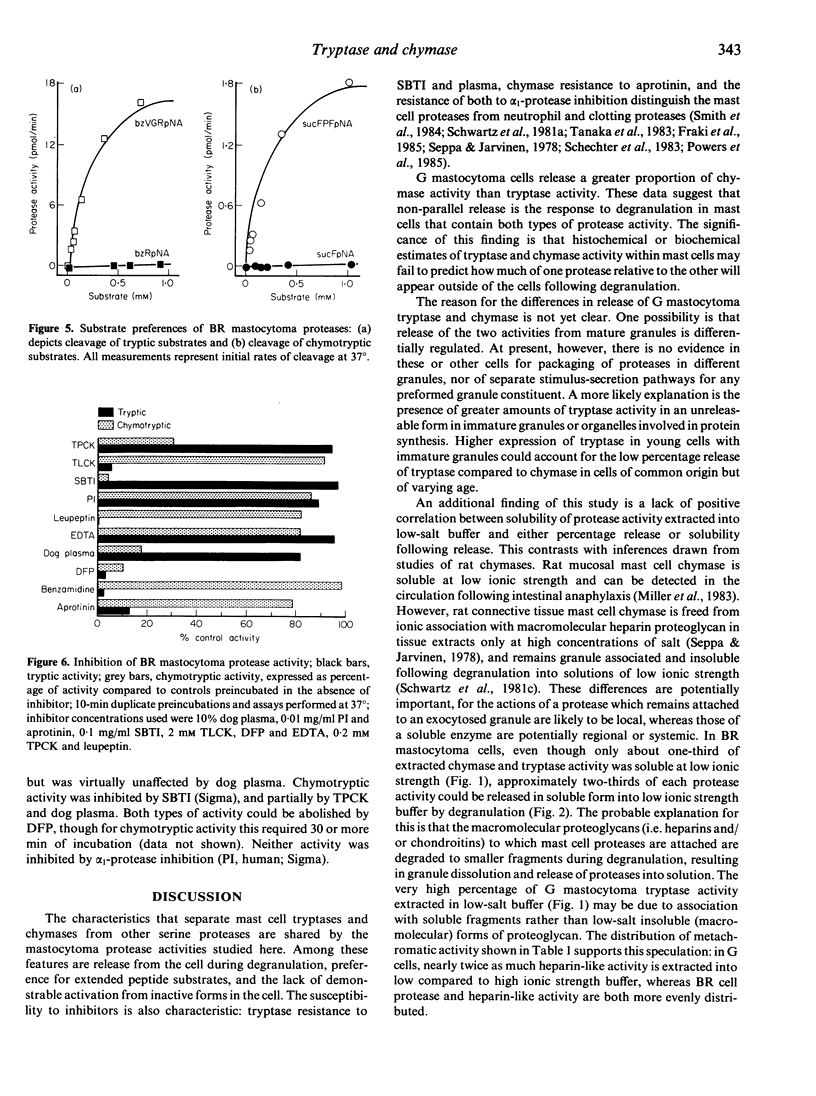
Mast cell secretory granules contain unique tryptic and chymotryptic serine proteases that differ between species and tissues. Direct comparison of these proteases in single-cell types has been hindered by the difficulty of obtaining adequate numbers of pure mast cells. In this study, we were able to compare tryptic and chymotryptic enzyme activity in cells of presumed monoclonal origin, using two stable lines ('BR' and 'G') of dog mastocytomas. The gel-filtration profiles, inhibitor susceptibilities and substrate preferences of tryptic and chymotryptic mastocytoma protease activities established their close resemblance to the tryptases and chymases of human and rodent mast cells. Striking heterogeneity was observed in the amounts and solubilities of the tryptic and chymotryptic activity in the two different mastocytoma cell lines. Incubation of cells from both lines with calcium ionophore A23187 caused non-cytotoxic release of protease activity. In contrast to chymase from rat connective tissue mast cells, protease activity that was insoluble after extraction at low ionic strength became soluble following ionophore-stimulated release. Neither tryptic nor chymotryptic activity was activated during degranulation, suggesting the absence of inactive precursors. Cells of the 'BR' line released both tryptic and chymotryptic activity in parallel with the granule marker histamine; cells of the 'G' line released a much smaller proportion of tryptic activity than of either chymotryptic activity or histamine. These differences in release of granule constituents from cells of common origin could be explained by developmental variations in the production of performed mediators or by differential regulation of preformed mediator release. We conclude that the differences in protease content, solubility and release in these mastocytoma lines are useful in evaluating the potential pathophysiological significance of the contribution of proteases to mast cell heterogeneity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Chiu H., Lagunoff D. Histochemical comparison of vertebrate mast cells. Histochem J. 1972 Mar;4(2):135–144. doi: 10.1007/BF01004972. [DOI] [PubMed] [Google Scholar]
- GLENNER G. G., COHEN L. A. Histochemical demonstration of a species-specific trypsin-like enzyme in mast cells. Nature. 1960 Mar 19;185:846–847. doi: 10.1038/185846a0. [DOI] [PubMed] [Google Scholar]
- Irani A. A., Schechter N. M., Craig S. S., DeBlois G., Schwartz L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques L. B., Wollin A. A modified method for the colorimetric determination of heparin. Can J Physiol Pharmacol. 1967 Sep;45(5):787–794. doi: 10.1139/y67-093. [DOI] [PubMed] [Google Scholar]
- Lazarus S. C., DeVinney R., McCabe L. J., Finkbeiner W. E., Elias D. J., Gold W. M. Isolated canine mastocytoma cells: propagation and characterization of two cell lines. Am J Physiol. 1986 Dec;251(6 Pt 1):C935–C944. doi: 10.1152/ajpcell.1986.251.6.C935. [DOI] [PubMed] [Google Scholar]
- Maier M., Spragg J., Schwartz L. B. Inactivation of human high molecular weight kininogen by human mast cell tryptase. J Immunol. 1983 May;130(5):2352–2356. [PubMed] [Google Scholar]
- Miller H. R., Woodbury R. G., Huntley J. F., Newlands G. Systemic release of mucosal mast-cell protease in primed rats challenged with Nippostrongylus brasiliensis. Immunology. 1983 Jul;49(3):471–479. [PMC free article] [PubMed] [Google Scholar]
- Powers J. C., Tanaka T., Harper J. W., Minematsu Y., Barker L., Lincoln D., Crumley K. V., Fraki J. E., Schechter N. M., Lazarus G. G. Mammalian chymotrypsin-like enzymes. Comparative reactivities of rat mast cell proteases, human and dog skin chymases, and human cathepsin G with peptide 4-nitroanilide substrates and with peptide chloromethyl ketone and sulfonyl fluoride inhibitors. Biochemistry. 1985 Apr 9;24(8):2048–2058. doi: 10.1021/bi00329a037. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Tewksbury D. A., Schechter N. M., Travis J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J Biol Chem. 1982 Aug 10;257(15):8619–8622. [PubMed] [Google Scholar]
- Sage H., Woodbury R. G., Bornstein P. Structural studies on human type IV collagen. J Biol Chem. 1979 Oct 10;254(19):9893–9900. [PubMed] [Google Scholar]
- Schechter N. M., Choi J. K., Slavin D. A., Deresienski D. T., Sayama S., Dong G., Lavker R. M., Proud D., Lazarus G. S. Identification of a chymotrypsin-like proteinase in human mast cells. J Immunol. 1986 Aug 1;137(3):962–970. [PubMed] [Google Scholar]
- Schechter N. M., Fräki J. E., Geesin J. C., Lazarus G. S. Human skin chymotryptic proteinase. Isolation and relation to cathepsin g and rat mast cell proteinase I. J Biol Chem. 1983 Mar 10;258(5):2973–2978. [PubMed] [Google Scholar]
- Schulman E. S., Kagey-Sobotka A., MacGlashan D. W., Jr, Adkinson N. F., Jr, Peters S. P., Schleimer R. P., Lichtenstein L. M. Heterogeneity of human mast cells. J Immunol. 1983 Oct;131(4):1936–1941. [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986 Jun 5;261(16):7372–7379. [PubMed] [Google Scholar]
- Schwartz L. B., Kawahara M. S., Hugli T. E., Vik D., Fearon D. T., Austen K. F. Generation of C3a anaphylatoxin from human C3 by human mast cell tryptase. J Immunol. 1983 Apr;130(4):1891–1895. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Austen K. F. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981 Nov 25;256(22):11939–11943. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Seldin D., Austen K. F. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981 Apr;126(4):1290–1294. [PubMed] [Google Scholar]
- Schwartz L. B., Riedel C., Caulfield J. P., Wasserman S. I., Austen K. F. Cell association of complexes of chymase, heparin proteoglycan, and protein after degranulation by rat mast cells. J Immunol. 1981 Jun;126(6):2071–2078. [PubMed] [Google Scholar]
- Seppä H. E., Järvinen M. Rat skin main neutral protease: purification and properties. J Invest Dermatol. 1978 Feb;70(2):84–89. doi: 10.1111/1523-1747.ep12541221. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Hougland M. W., Johnson D. A. Human lung tryptase. Purification and characterization. J Biol Chem. 1984 Sep 10;259(17):11046–11051. [PubMed] [Google Scholar]
- Tanaka T., McRae B. J., Cho K., Cook R., Fraki J. E., Johnson D. A., Powers J. C. Mammalian tissue trypsin-like enzymes. Comparative reactivities of human skin tryptase, human lung tryptase, and bovine trypsin with peptide 4-nitroanilide and thioester substrates. J Biol Chem. 1983 Nov 25;258(22):13552–13557. [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Structure, specificity and localization of the serine proteases of connective tissue. FEBS Lett. 1980 Jun 2;114(2):189–196. doi: 10.1016/0014-5793(80)81112-4. [DOI] [PubMed] [Google Scholar]


