Abstract
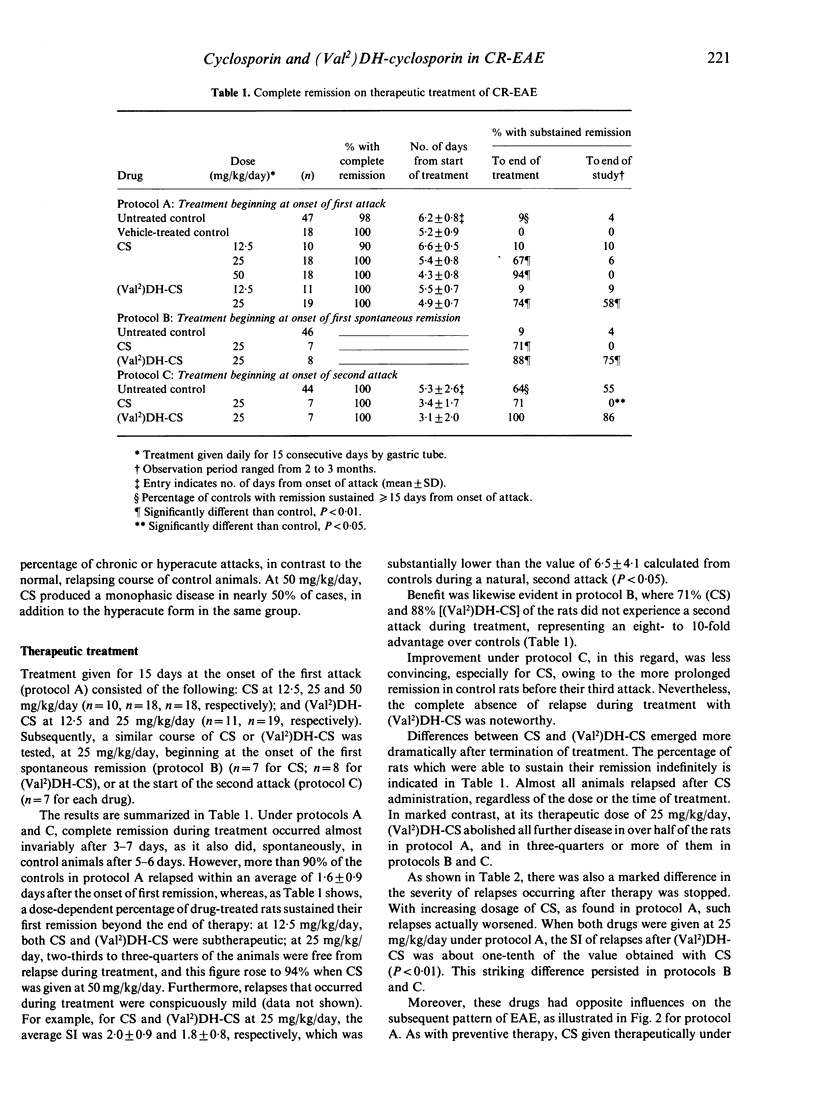
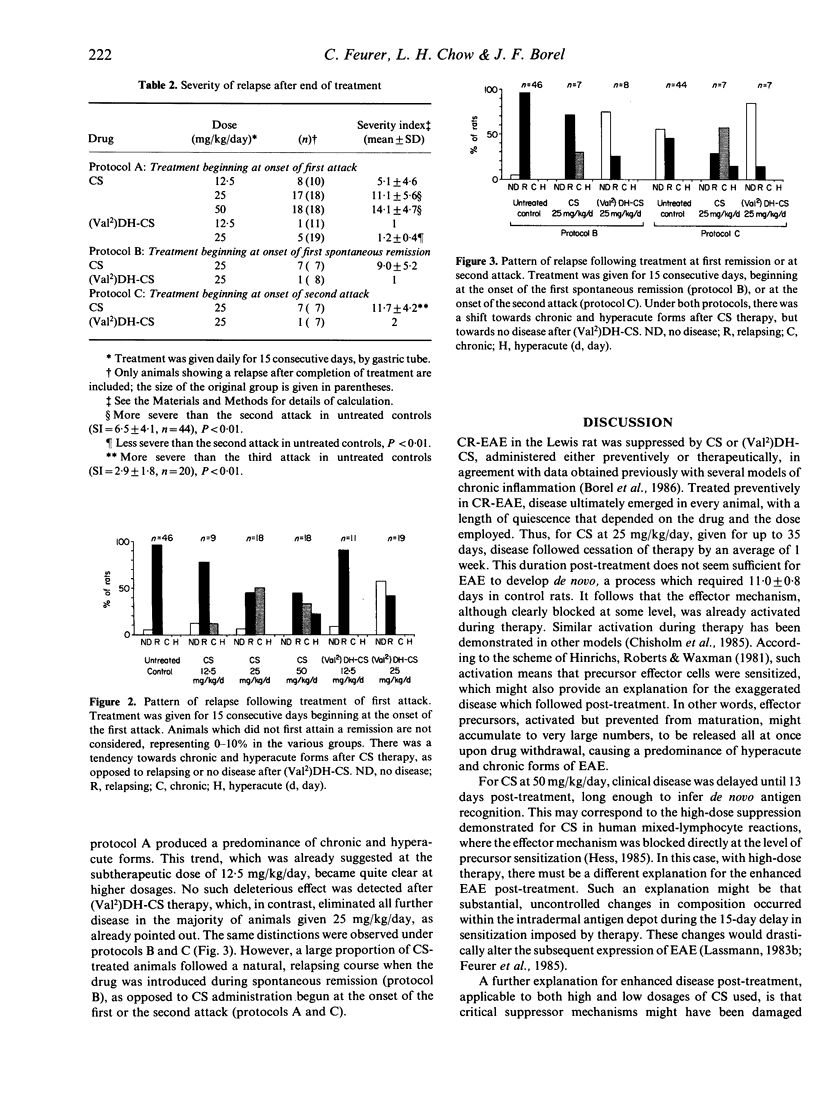
Cyclosporin (CS) and valine2-dihydro-cyclosporin [(Val2)DH-CS] were tested in adult Lewis rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE), induced by the immunization of guinea-pig spinal cord emulsified in complete Freund's adjuvant. The drugs were given orally for 15 or 35 days, at 12.5, 25 or 50 mg/kg/day, starting either on the day of sensitization (preventive treatment) or at one of three subsequent times (therapeutic treatment): the onset of the first attack (protocol A); the onset of the first spontaneous remission (protocol B); and the onset of the second attack (protocol C). Used therapeutically in protocol A, at doses above 12.5 mg/kg/day, both drugs prolonged remission past the end of therapy in more than two-thirds of the treated animals, compared to less than 10% of controls. Trends were similar under protocols B and C. Disease developing after preventive treatment with either drug was predominated by chronic and hyperacute attacks, in contrast to the relapsing course of controls. This pattern was also the result after CS was given therapeutically, whereas (Val2)DH-CS in such circumstances eliminated all further attacks in the majority of rats (58-86% at 25 mg/kg/day) and only minimal disease occurred in the remainder. We conclude that both drugs, in this model, are beneficial during administration; however, in contrast to CS, (Val2)DH-CS possesses an important, curative action when applied therapeutically.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. Clinical transplantation--overview. Prog Allergy. 1986;38:269–292. [PubMed] [Google Scholar]
- Bolton C., Allsopp G., Cuzner M. L. The effect of cyclosporin A on the adoptive transfer of experimental allergic encephalomyelitis in the Lewis rat. Clin Exp Immunol. 1982 Jan;47(1):127–132. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Gunn H. C. Cyclosporine as a new approach to therapy of autoimmune diseases. Ann N Y Acad Sci. 1986;475:307–319. doi: 10.1111/j.1749-6632.1986.tb20879.x. [DOI] [PubMed] [Google Scholar]
- Cammisuli S., Feurer C. The effect of cyclosporin-A and dihydrocyclosporin-D on the therapy and prophylaxis of experimental allergic encephalomyelitis. Prog Clin Biol Res. 1984;146:415–421. [PubMed] [Google Scholar]
- Chisholm P. M., Drayson M. T., Cox J. H., Ford W. L. The effects of cyclosporin on lymphocyte activation in a systemic graft-vs.-host reaction. Eur J Immunol. 1985 Oct;15(10):1054–1059. doi: 10.1002/eji.1830151018. [DOI] [PubMed] [Google Scholar]
- Feurer C., Prentice D. E., Cammisuli S. Chronic relapsing experimental allergic encephalomyelitis in the Lewis rat. J Neuroimmunol. 1985 Dec;10(2):159–166. doi: 10.1016/0165-5728(85)90006-2. [DOI] [PubMed] [Google Scholar]
- Hiestand P. C., Gunn H. C., Gale J. M., Ryffel B., Borel J. F. Comparison of the pharmacological profiles of cyclosporine, (Nva2)-cyclosporine and (Val2)dihydro-cyclosporine. Immunology. 1985 Jun;55(2):249–255. [PMC free article] [PubMed] [Google Scholar]
- Hinrichs D. J., Roberts C. M., Waxman F. J. Regulation of paralytic experimental allergic encephalomyelitis in rats: susceptibility to active and passive disease reinduction. J Immunol. 1981 May;126(5):1857–1862. [PubMed] [Google Scholar]
- Hinrichs D. J., Wegmann K. W., Peters B. A. The influence of cyclosporin A on the development of actively induced and passively transferred experimental allergic encephalomyelitis. Cell Immunol. 1983 Apr 1;77(1):202–209. doi: 10.1016/0008-8749(83)90020-5. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Chronic relapsing experimental allergic encephalomyelitis: its value as an experimental model for multiple sclerosis. J Neurol. 1983;229(4):207–220. doi: 10.1007/BF00313549. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., McFarland H. F. Multiple sclerosis (second of two parts). N Engl J Med. 1982 Nov 11;307(20):1246–1251. doi: 10.1056/NEJM198211113072005. [DOI] [PubMed] [Google Scholar]
- Reiber H., Suckling A. J. Cyclosporin-A treatment of experimental allergic encephalomyelitis: changes in immunological regulation and blood-CSF barrier function. J Neuroimmunol. 1986 Aug;12(2):121–130. doi: 10.1016/0165-5728(86)90025-1. [DOI] [PubMed] [Google Scholar]
- Welch A. M., Holda J. H., Swanborg R. H. Regulation of experimental allergic encephalomyelitis. II. Appearance of suppressor cells during the remission phase of the disease. J Immunol. 1980 Jul;125(1):186–189. [PubMed] [Google Scholar]
- Wiśniewski H. M., Keith A. B. Chronic relapsing experimental allergic encephalomyelitis: an experimental model of multiple sclerosis. Ann Neurol. 1977 Feb;1(2):144–148. doi: 10.1002/ana.410010207. [DOI] [PubMed] [Google Scholar]



