Abstract
Tet(O) is an elongation factor-like protein which confers resistance to the protein synthesis inhibitor tetracycline by promoting the release of the drug from its inhibitory site on the ribosome. Here we investigated the interaction of Tet(O) with the elongating ribosome and show, using dimethyl sulfate (DMS) probing and binding assays, that it interacts preferentially with the post-translocational ribosome. Furthermore, using an XTP-dependent mutant of Tet(O), we demonstrated that Tet(O) induces conformational rearrangements within the ribosome which can be detected by EF-Tu, and manifested as a stimulation in the GTPase activity of this elongation factor. As such, these conformational changes probably involve the ribosomal GTPase-associated center and, accordingly, Tet(O) alters the DMS modification pattern of the L11 region. Additionally, tetracycline binding is associated with an Ea of 58 kJ/mol. These results suggest a model where both Tet(O) and tetracycline induce a conformational change in functionally opposite directions and the Tet(O)-induced conformation persists after it has left the ribosome; this prevents rebinding of the drug while allowing productive A-site occupation by a ternary complex in the presence of tetracycline.
Keywords: antibiotic resistance/protein synthesis/ribosome/Tet(O)/tetracycline
Introduction
Tetracycline resistance in the intestinal pathogen, Campylobacter jejuni, can be conferred by a soluble protein factor called Tet(O) (Manavathu et al., 1990). Tet(O) is part of a larger group of proteins called ribosomal protection proteins (RPPs), which includes Tet(M), Tet(Q), Tet(S), Tet(T), Tet(W) and OtrA (Chopra and Roberts, 2001). RPPs share extensive sequence homology with ribosome-binding proteins involved in protein synthesis (Sanchez-Pescador et al., 1988) and, based on the presence of conserved motifs, can be grouped within the translation factor superfamily of GTPases (Leipe et al., 2002). As such, Tet(O) interacts directly with the target of tetracycline, the 70S ribosome, and promotes the release of tetracycline in a GTP-dependent manner (Burdett, 1996; Trieber et al., 1998).
Tetracycline inhibits protein synthesis, where it specifically blocks the elongation cycle by preventing incoming aminoacyl-tRNA (aa-tRNA) from binding to the ribosomal A-site. Recently, X-ray crystallographic studies of a tetracycline–ribosome complex revealed a tetracycline-binding site that is positioned so as to interfere sterically with A-site binding (Brodersen et al., 2000; Pioletti et al., 2001). Accordingly, recent dimethyl sulfate (DMS) probing experiments demonstrated that Tet(O) removes tetracycline bound specifically to this site (Connell et al., 2002), consistent with the idea that this site represents the single inhibitory site on the 30S subunit (Tritton, 1977).
Current models based on cryo-electron microscopy (EM) reconstructions propose that Tet(O) does not interfere directly with tetracycline binding, but rather acts allosterically, distorting the tetracycline-binding site and thus releasing the bound drug (Spahn et al., 2001). The proposed conformational change resulting in tetracycline release probably involves helix 34 (h34) of the 16S rRNA as: (i) h34 forms an integral part of the primary tetracycline-binding site (Brodersen et al., 2000; Pioletti et al., 2001); (ii) cryo-EM reconstructions show that domain IV of Tet(O) contacts the base of h34 (Spahn et al., 2001); and (iii) Tet(O) protects nucleotides at the base of h34 from chemical modification by DMS (Connell et al., 2002). It should be noted, however, that the interaction of Tet(O) is not limited to h34. Cryo-EM data suggest that it also makes contact with the ribosomal protein S12 and helices h5/18 of the small ribosomal subunit, while on the large subunit it contacts helices H43/44/95 (Spahn et al., 2001).
In spite of this rather detailed knowledge, several basic aspects of the mechanism of Tet(O) action have still to be elucidated. In this study, we first determine at which point Tet(O) is intercalating into the elongation cycle, showing that Tet(O) preferentially interacts with the post-translocational ribosome. Furthermore, we analyze the interaction of Tet(O) with the GTPase-associated region (GAR) on the ribosome, finding that Tet(O) displays a distinctive interaction with the L11 region and is capable of inducing conformational changes within the ribosome. The interaction of tetracycline with the ribosome has also been studied, revealing that the rate-limiting step of binding follows first order reaction kinetics and is accompanied by a significant activation energy. These results are incorporated into a model that describes the entire cycle of Tet(O)-mediated tetracycline resistance.
Results
Chemical probing of Tet(O) functional complexes
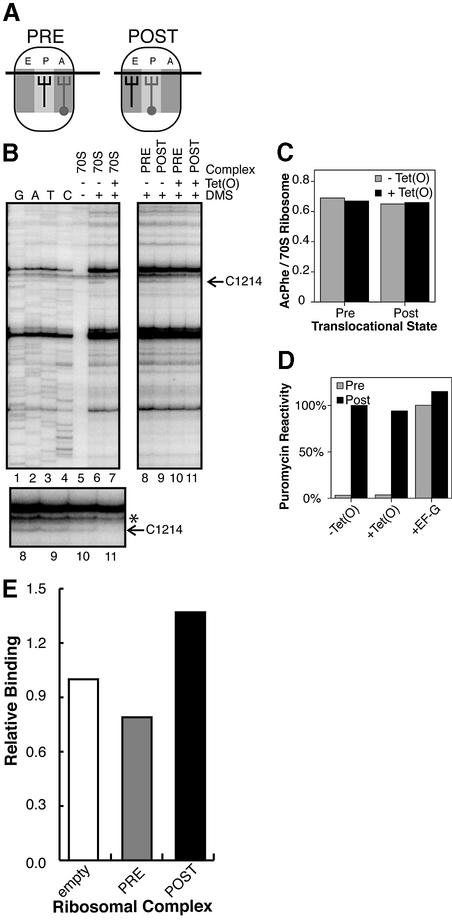
Prior studies showed that there is likely to be a direct interaction between Tet(O) and the small ribosomal subunit that is important for triggering the release of tetracycline, and is revealed as a characteristic Tet(O)-dependent protection from DMS modification at the base of h34 (C1214) in the 16S rRNA (Connell et al., 2002). Here we use this protection to monitor the interaction of Tet(O) with the ribosome in various functional states. Tet(O) was bound to pre- and post-translocational (PRE and POST) ribosomal complexes where the PRE complex contains a deacyl-tRNAfMet in the P-site and an AcPhe-tRNAPhe in the A-site (Figure 1A). This complex subsequently was subjected to EF-G-dependent translocation to generate a POST complex such that the AcPhe has shifted to the P-site and the deacyl-tRNAfMet to the E-site (Figure 1A). Tet(O) was bound to these ribosomal complexes in the presence of a non-hydrolyzable GTP analog (GMPPNP) which allows for formation of a stable 70S·Tet(O)·GMPPNP complex (Trieber et al., 1998). Subsequently, these complexes were treated with DMS, which modifies adenosine and cytidine at their N1 and N3 position, respectively, depending on their chemical environment, in a manner that can be readily detected by primer extension analysis (Stern et al., 1988). The results of a typical primer extension experiment are shown in Figure 1B. C1214 in the POST state ribosome was strongly protected from DMS modification in the presence of Tet(O) (Figure 1B; compare lanes 9 and 11), while that of the PRE state ribosome was only weakly affected by Tet(O) (Figure 1B; compare lanes 8 and 10). When several gels are quantified, C1214 in the POST state ribosome–Tet(O) complex (Figure 1B; lane 11) was 51 ± 13% as reactive as it was in the POST complex alone (Figure 1B; lane 9). In contrast, when Tet(O) was incubated with ribosomes in the PRE state (Figure 1B; lane 10), the reactivity of C1214 was relatively unchanged, as it was 98 ± 26% as reactive as in the PRE state ribosome alone (Figure 1B; lane 8). This shows that Tet(O) preferentially interacts with the POST state ribosome, shielding C1214:N3 from methylation, but does not interact correspondingly with the PRE state ribosome.
Fig. 1. (A) Pre- and post-translocational states: in the PRE complex, deacyl-tRNAfMet is located in the P-site and AcPhe-tRNAPhe is located in the A-site, whereas in the POST state the tRNAs have been translocated to the E- and P-sites, respectively. (B) DMS reactivity of 16S rRNA isolated from pre- and post-translocational complexes: a magnified view of the region around C1214 (indicated with an arrow) is shown and the lanes are numbered exactly as in the upper gel. The dideoxy sequencing lanes are indicated with G, A, T and C, whereas the components in the other reactions are indicated above the gel (70S, empty ribosomes; PRE, pre-translocational state ribosomes; POST, post-translocational state ribosomes). The gel shown is representative of that seen in four primer extension experiments made from two independent complexes. The intensity of the bands was normalized using the band indicated (*). (C) Effect of Tet(O) on AcPhe-tRNAPhe binding: the amount of AcPhe-tRNAPhe bound to the pre- and post-translocational states in the presence (black bars) and absence of Tet(O) (gray bars) is shown. The PRE and POST complexes were established as described in Materials and methods, and subsequently Tet(O) was bound to them in the presence of GMPPNP under the same conditions as described for the DMS probing experiments. AcPhe-tRNAPhe binding is given as the ratio of pmol of [14C]AcPhe-tRNAPhe bound per pmol of 70S ribosomes. (D) Effect of Tet(O) on the puromycin reactivity of the prepared complexes: the puromycin reactivity of the complexes used in the chemical probing experiments is indicated, where, in addition, EF-G was added to PRE and POST complexes to illustrate the change in puromycin reactivity that accompanies translocation of the tRNAs. The gray bars represent PRE state complexes, and the black bars are POST state complexes, where EF-G and Tet(O) are added as indicated on the x-axis. The PRE and POST complexes were established as described in Materials and methods, and subsequently Tet(O) and EF-G were bound to them in the presence of GMPPNP under the same conditions as described for the DMS probing experiments. The puromycin (PM) reactivity is given relative to that of the POST complex, which corresponds to 0.6 pmol of AcPhe-PM per pmol 70S ribosome, and is given as a percentage. (E) Binding of Tet(O)HN to empty ribosomes, PRE state ribosomes or POST state ribosomes: Tet(O) binding (as indicated by the co-elution of [35S]GTPγS and ribosomes) to the pre- and post-translocational ribosomal complexes (gray and black bars, respectively) is shown relative to the binding of Tet(O) to the empty ribosomes. Tet(O)HN was bound to the functional complexes (Materials and methods), and the complex isolated on cDNA spun columns (Pharmacia) essentially as described previously (Trieber et al., 1998), where the binding reaction contained 1.6 µM Tet(O)HN, 1.6 µM ribosomal complex and 25 µM [35S]GTPγS as indicated. The d.p.m. values have been normalized based on the amount of ribosomes eluted in the first fraction. Additionally, the background values derived from the amount of GTPγS that elutes with either Tet(O) or ribosomes alone have been subtracted. The results are representative of two experiments, where the duplicate determinations deviate from the mean by <11%.
In order to confirm that the PRE and POST complexes were not altered by the presence of Tet(O), we analyzed the effect of Tet(O) on the level of tRNA binding and on the puromycin reactivity of the AcPhe-tRNAPhe (Figure 1C and D). In Figure 1C, it can be seen that when Tet(O) was bound to the PRE and POST complexes under the same conditions as in the DMS probing experiments, it had no effect on the level of AcPhe-tRNAPhe binding. Similarly, Tet(O) was unable to alter the location of the tRNA and, accordingly, had no effect on the puromycin reactivity of the ribosomal complexes, unlike EF-G, which can translocate the tRNAs, resulting in the formation of a POST complex indicated by an increase in the puromycin reactivity (Figure 1D). These results indicate that in the DMS probing experiments, we are indeed detecting the interaction of Tet(O) with defined PRE and POST complexes and that the change in C1214 DMS reactivity is due exclusively to the action of Tet(O) rather than to changes in tRNA occupation or location. It is also interesting to note that when Tet(O) is locked on the POST state ribosome using GMPPNP, it does not inhibit the puromycin reaction. Moreover, the fact that Tet(O) does not translocate the tRNA or interact with the PRE complex clearly explains why Tet(M), a RPP like Tet(O), can not substitute for EF-G in vitro or in vivo (Burdett, 1991, 1996), despite their overall sequence similarity.
Tet(O) binding to PRE and POST complexes
In the above DMS probing experiments, we made the assumption that the Tet(O)-dependent protection of C1214 from DMS modification is an indication of Tet(O) binding to the ribosome. To confirm this assumption, we directly measured Tet(O) binding to PRE and POST complexes by following their co-elution from a size exclusion column. When Tet(O) and [35S]GTPγ were applied to the column in the presence of either empty ribosomes, a PRE complex or a POST complex, we observed that 1.4–1.7 times more GTPγS is co-eluted with the POST complex as compared with empty ribosomes or with the PRE complex, indicating that Tet(O) binds preferentially to the POST state. The occupancy of the ribosomal complex in the first fraction from the size exclusion column as judged by the co-elution of GTPγS and 70S ribosomes corresponded to 0.59, 0.47 and 0.81 pmol Tet(O) per pmol 70S ribosome in the empty, PRE and POST complexes, respectively. In this case, it is especially significant that the POST–Tet(O) complex displayed a 35% higher occupancy compared with the empty ribosome–Tet(O) complex because this corresponds to the fraction of ribosomes in a defined POST state (see Materials and methods). This implies that in the sample of POST state ribosomes, where only 30% are in a defined POST state, all of these are occupied by Tet(O) because the amount of Tet(O) used was not saturating [1:1 Tet(O) to ribosome ratio]. By the same reasoning, the similar binding of Tet(O) to pre-translocational ribosomes and empty ribosomes suggests that Tet(O) is only binding to the empty ribosome fraction in the PRE sample. These results support the chemical probing experiments above and establish that Tet(O) preferentially interacts with the post-translocational ribosome and, in this sense, the absence of a Tet(O)-dependent footprint on C1214 in the pre-translocational state probably results from a lack of Tet(O) binding.
Interplay of Tet(O)D131N with EF-Tu in the presence of ribosomes
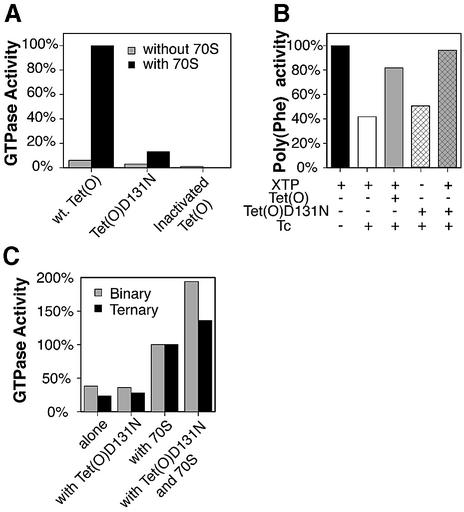
Previous studies employing a xanthosine triphosphate (XTP)-dependent mutant of EF-Tu have been especially useful for studying the interaction of the elongation factors by following their (G/X)TPase activity (Hwang and Miller, 1987; Weijland and Parmeggiani, 1993; Weijland et al., 1994). With a similar aim, we constructed an XTP-dependent mutant of Tet(O) using a D131N mutation homologous to the D138N mutation in EF-Tu that abolished the affinity of protein for GDP while increasing its affinity for XDP (Hwang and Miller, 1987; Weijland et al., 1994). Accordingly, in vitro studies showed that Tet(O)D131N has very low ribosome-stimulated GTPase activity, compared with that of wild-type Tet(O) (Figure 2A), indicating that Tet(O)D131N has lost its ability to hydrolyze GTP efficiently. In line with this observation, Tet(O)D131N was unable to confer tetracycline resistance in vivo (data not shown), since GTPase activity is essential for Tet(O) (Grewal et al., 1993) and XTP is not present within Escherichia coli (Hwang and Miller, 1987). Additionally, when Tet(O)D131N was added to a tetracycline-inhibited poly(Phe) system, it was able to relieve inhibition only when XTP was present (Figure 2B; compare white and gray cross-hatched bars), indicating that it is dependent on XTP. Note that the poly(Phe) synthesis system contains GTP, indicating that Tet(O)D131N is no longer active in the presence of GTP and absence of XTP.
Fig. 2. (A) Intrinsic and ribosome-stimulated GTPase activity of Tet(O)D131N: the intrinsic GTPase activity (gray bars) and ribosome-stimulated GTPase activity (black bars) of Tet(O), Tet(O)D131N and heat-inactivated Tet(O) (95°C, 10 min) are shown. One hundred percent GTPase activity corresponds to the ribosome-stimulated GTPase activity of wild-type Tet(O) [2800 pmol GTP hydrolyzed per pmol of Tet(O) at 37°C over 10 min]. (B) Activity of Tet(O)D131N in a tetracycline-inhibited poly(Phe) assay: the ability of Tet(O) and Tet(O)D131N to rescue a tetracycline (25 µM)-inhibited poly(Phe) synthesizing system is shown. The activity of the uninhibited system (24 pmol of [14C]Phe incorporated per ribosome in 60 s at 37°C) is indicated with the black bar. The composition of the other reactions is indicated below the graph, and the activity of these reactions is given relative to the uninhibited system. The assay used was a modified version of that described previously (Bommer et al., 1996). (C) Stimulation of the EF-Tu-dependent GTPase activity by Tet(O)D131N: the effect of Tet(O)D131N on the ribosome-stimulated GTPase activity of the binary (EF-Tu·GTP; shaded bars) or ternary (EF-Tu·Phe-tRNA·GTP; black bars) complex is shown. The reported GTPase activities are given relative to the ribosome-stimulated GTPase activity of the binary and ternary complexes (0.6 and 2.7 pmol GTP hydrolyzed in 5 min at 37°C, respectively).
The two ribosomal elongation factors, EF-G and EF-Tu, cooperatively stimulate each other’s GTPase activity in the presence of empty ribosomes (Mesters et al., 1994). Does Tet(O) as a derivative of EF-G do the same? To measure clearly a possible change in the GTPase activity of EF-Tu in the presence of Tet(O) and 70S ribosomes, the Tet(O)D131N mutant was used because the ribosome-stimulated GTPase activity of wild-type Tet(O) is much larger than that of EF-Tu; the D131N mutant will consume XTP, so the measured GTPase will be exclusive to EF-Tu. The GTPase activity of EF-Tu was studied using isolated binary (EF-Tu·GTP) or ternary (EF-Tu·Phe-tRNA·GTP) complexes on their own or in the presence of 70S ribosomes and/or Tet(O)D131N plus XTP. In Figure 2C, it can be seen that on their own, the binary and ternary complexes have a low GTPase activity, and the activity is unchanged by the addition of Tet(O)D131N. This indicates that there is no direct interaction between the two that affects the GTPase activity when free in solution. When either the binary or ternary complex were mixed with 70S ribosomes, there was an ∼3-fold increase in the GTPase activity. This ribosome-stimulated GTPase activity was enhanced a further 1.4–1.9 times by the addition of Tet(O)D131N to the ternary and binary complex, respectively. The increase in ribosome-stimulated EF-Tu GTPase activity by Tet(O)D131N suggests that Tet(O) invokes a conformational change in the ribosome that persists after it has left the ribosome, thus leading to a stimulation in EF-Tu’s ribosome-dependent GTPase activity, similar to what was suggested for the synergy between EF-G and EF-Tu (Mesters et al., 1994). It is unlikely that the increase in GTPase activity is due to residual GTPase activity in the Tet(O)D131N mutant, as the concentration of free GTP in the solution is low; and what does dissociate from the isolated EF-Tu would have to compete with the large excess of free XTP for binding to Tet(O). Furthermore, the same amount of Tet(O)D131N was present in the reactions containing either binary or ternary complex, but the extent of GTP hydrolysis was different in these reactions, indicating that GTP hydrolysis was not caused by the residual GTPase activity of Tet(O)D131N.
Defining the interaction between Tet(O) and the ribosomal GTPase-associated center
The stimulatory effect of Tet(O) on EF-Tu may be mediated by the GAR (L11 region) and the sarcin-ricin loop (SRP) on the 50S ribosomal subunit. Since both elements have been proposed to coordinate elongation factor binding (Nierhaus et al., 1992; Wool et al., 1992; Porse et al., 1998; Wimberly et al., 1999), they could play a role in the selective binding of Tet(O) to the POST state ribosome.
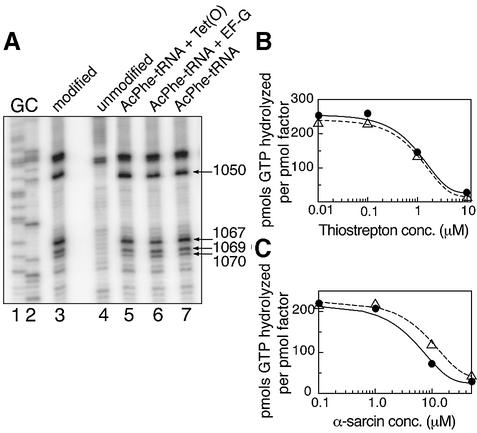
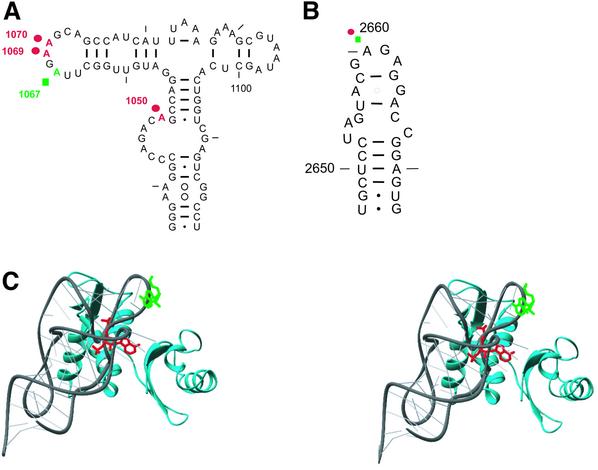
DMS probing and primer extension analysis of the rRNA elements in the GAR show that Tet(O) interacts with the L11 region, namely H42/43/44 (Figure 3A). In this region, Tet(O) shows a unique interaction compared with that already described for EF-G (Moazed et al., 1988). Tet(O) reduced the DMS reactivity of A1070 to 75% (± 4%) of its respective reactivity in the empty ribosome while reducing that of A1069 to 84% (± 7%); in contrast, EF-G decreased the DMS reactivity of A1067 to 79% (± 5%) (Figure 3A). Tet(O) also differs from EF-G in that it interacts with H42 where it enhanced the accessibility of A1050 such that its DMS reactivity increased to 136% (± 10%) (Figure 3A). Furthermore, the presence of AcPhe-tRNAPhe had no effect on the DMS reactivity of the rRNA scanned (Figure 3A), and therefore the alterations in DMS reactivity observed above are due exclusively to the added protein. Additionally, scanning by reverse transcription detected changes in the DMS modification pattern within H95 (protection of A2660; data not shown) such that the Tet(O)·70S complex showed a pattern very similar to that already described for the EF-G·70S ribosome complex (Moazed et al., 1988). These results illustrate that the interaction with the GAR, although similar to that of the EF-G (Moazed et al., 1988), is unique to Tet(O), a feature that could be important for coordinating the interaction of Tet(O) with the elongating ribosome or be responsible for Tet(O)’s inability to promote translocation.
Fig. 3. (A) DMS probing of the L11 region in the 23S rRNA: the PhosphorImager scans show the primer extension analysis of H42/43/44 where the template used was unmodified rRNA (lane 4) or DMS-modified rRNA from 70S ribosomes (lane 3); lanes 5–7, the additions to programmed 70S ribosomes are given above each lane. The dideoxy sequencing reactions are labeled G and C. Primer extension was carried out using an oligonucleotide complementary to positions 1120–1136 of the 23S rRNA. Positions that experience changes in DMS reactivity are marked with arrows. This gel of the L11 region is representative of three primer extension experiments carried out on independent complexes. (B and C) Effect of thiostrepton and α-sarcin on the GTPase activity of Tet(O): the effect of an increasing concentration of thiostrepton (B) and α-sarcin (C) on the GTPase activity of Tet(O) (filled circles) and EF-G (open triangles) is shown.
To establish that these detected interactions have functional significance for Tet(O), we monitored Tet(O) activity in the presence of thiostrepton and α-sarcin. These compounds target the L11 region and α-sarcin loop and presumably inhibit EF-G’s GTPase activity by preventing EF-G from interacting stably with the ribosome (Bodley et al., 1970; Pestka, 1970; Fernandez-Puentes and Vazquez, 1977; Hausner et al., 1987; Cameron et al., 2002). As demonstrated in Figure 3B, thiostrepton blocked the ribosome-stimulated GTPase of Tet(O) in a fashion similar to EF-G; both proteins lost 50% of their activity when thiostrepton was present at 1 µM. Thiostrepton sensitivity has also been observed with Tet(M), a homolog of Tet(O) sharing 76% amino acid identity, and was shown to result from the disruption of the Tet(M)–ribosome interaction (Dantley et al., 1998), suggesting that the interaction with the L11 region detected by DMS probing is an important determinant in Tet(O)/(M) binding. When 70S ribosomes were treated with increasing amounts of α-sarcin, a ribotoxin that clips the phosphodiester backbone in H95 3′ to G2661 (Endo and Wool, 1982; Hausner et al., 1987), they became increasingly defective in stimulating the GTPase activity of Tet(O) and EF-G (Figure 3C); 50% of their activity was lost at 7 and 12 µM α-sarcin, respectively. The sensitivity of Tet(O)’s ribosome-dependent GTPase activity to thiostrepton and α-sarcin suggests that the interactions between Tet(O) and the 23S rRNA (H95 and H42/43/44) detected above are important for Tet(O) activity and may regulate its binding in a way similar to that seen with EF-G (Hausner et al., 1987; Cameron et al., 2002).
Effect of temperature on tetracycline binding
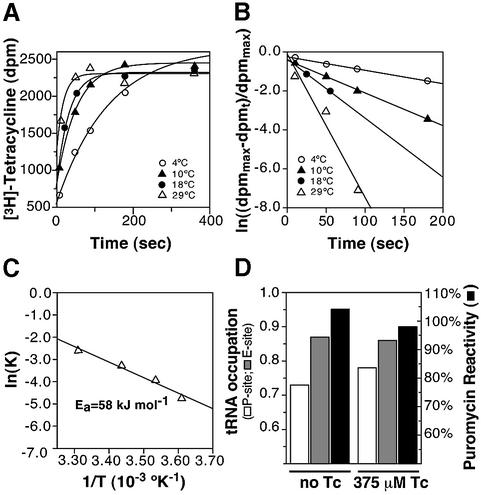
Tet(O) and tetracycline both seemingly affect h44 in the 16S rRNA (Dahlberg et al., 1973; Connell et al., 2002). To explore the possibility that tetracycline binding is accompanied by structural rearrangements, we calculated the activation energy for tetracycline binding. The kinetics of tetracycline binding to 70S ribosomes were followed at various temperatures for times up to 60 min but, as seen in Figure 4A, the binding saturated after ∼180 s. As the binding kinetics in Figure 4A showed a clear temperature dependence, they were processed to derive a value for the activation energy (Ea) for tetracycline binding. This involved the initial assumption that the binding reaction is second order, with a rate-limiting first order step such as a conformational change following equation 1 (Materials and methods). As seen in Figure 4B, this assumption indeed holds true as the plot of ln[(d.p.m.max – d.p.m.t)/d.p.m.max] versus time yielded straight lines, where the slope corresponds to the initial apparent rate constant. As illustrated in Figure 4C, the temperature dependence of the rate constants was then determined using the Arrhenius equation (equation 2), and an Ea of 58 kJ/mol was derived from the slope of the resulting line.
Fig. 4. Kinetics of tetracycline binding. (A) The the reaction kinetics for tetracycline binding to the ribosome are shown at 4°C (open circles), 10°C (filled triangles), 18°C (filled circles) and 29°C (open triangles). (B) The data from binding kinetics described in (A) are processed according to equation 1 and plotted as ln[(dpmmax – dpmt)/dpmmax] versus time. The slopes of the straight lines give the apparent rate constants: k4 = 0.0088/s (4°C), k10 = 0.020/s (10°C), k18 = 0.038/s (18°C) and k29 = 0.0748/s (29°C). (C) The derived rate constants are plotted according to the Arrhenius equation (equation 2), and the resulting activation energy amounts to 58 kJ/mol. (D) The effect of 375 µM tetracycline on the occupation of the ribosomal P-site by [14C]AcPhe-tRNAPhe (white bars), on the occupation of the E-site by [32P]tRNA (gray bars) and on the puromycin reactivity of the P-site-bound [14C]AcPhe-tRNAPhe (black bars). The tRNA occupation is given as the amount of tRNA bound per ribosome, and the puromycin reactivity represents the percentage of P-site-bound tRNA that is puromycin reactive.
The binding of aa-tRNA to the A-site of a post-translocational ribosome triggers the release of deacylated tRNA from the E site (Rheinberger and Nierhaus, 1986). Tetracycline, like the tRNA, binds to the decoding component of the ribosomal A-site (Brodersen et al., 2000; Pioletti et al., 2001); therefore, we investigated the possibility that the activation energy associated with tetracycline binding is used similarly to release the E-site-bound tRNA. For this reason, a POST complex was constructed in the presence of MF-mRNA, with a deacylated [32P]tRNAfMet at the E-site and an Ac[14C]Phe-tRNA at the P-site. As clearly seen in Figure 4D, tetracycline had no effect on [32P]tRNA occupation of the E-site (gray bars). Additionally, tetracycline had no effect on the AcPhe-tRNAPhe bound to the P-site (white bars), nor on its puromycin reactivity (black bars), indicating that tetracycline is not shifting the tRNA between various binding sites. These results indicate that although tetracycline does bind to the A-site, it does not mimic an acylated tRNA in its ability to trigger tRNA release from the E-site.
Discussion
Here we present two lines of evidence to show that Tet(O) interacts with the post-translocational ribosome, and thus demonstrate, for the first time, the step at which the ribosomal protection proteins interact with the elongation cycle. Additionally, we substantiate the observations made by cryo-EM (Spahn et al., 2001) that Tet(O) interacts with the GAR region on the 50S subunit, and extended these studies by identifying the specific bases in the rRNA whose local environment changes upon Tet(O) binding. Moreover, we present evidence that Tet(O) is able to alter the conformation of the ribosome. These results broaden our knowledge of Tet(O) action, which previously was limited only to the step of actual tetracycline release, and here we present a model detailing the complete cycle of Tet(O)-mediated tetracycline resistance.
Chemical probing and binding experiments clearly demonstrate that Tet(O) interacts with and binds to the post-translocational ribosome. The interaction of Tet(O) with C1214 near the primary tetracycline-binding site (Brodersen et al., 2000; Pioletti et al., 2001), in the post-translocational ribosome, is consistent with the role of Tet(O) in conferring tetracycline resistance, since tetracycline arrests the elongation cycle in the post-translocational state by blocking A-site occupation. It is interesting to note that Tet(O) has been footprinted on several ribosomal complexes, i.e. empty 70S, 70S with AcPhe-tRNAPhe in the P-site, and POST complexes, all of which have an empty A-site, and it is only the PRE complex, with an occupied A-site, that fails to be engaged by Tet(O) (Connell et al., 2002; this study). Cryo-EM reconstructions (Spahn et al., 2001) do, in fact, suggest that domain IV of Tet(O) and the A-site-bound tRNA are in close proximity. Regulation based on A-site occupation is logical since tetracycline’s role is to block A-site occupation and, as such, the prolonged pause of the ribosome with an open A-site could provide the kinetic opportunity for Tet(O) to interact with the ribosome. Additionally, a tetracycline-associated conformational change (Dahlberg et al., 1973; Tritton, 1977; Noah et al., 1999) could also serve as a recognition determinant to promote Tet(O) binding and subsequent tetracycline release.
In this study, we also identify the α-sarcin loop and the L11 region as important determinants in Tet(O) activity by showing that Tet(O) alters the DMS reactivity of specific rRNA bases in these regions, and that compounds (thiostrepton and α-sarcin) which target these regions inhibit the ribosome-dependent GTPase activity of Tet(O). These same regions are important for the binding of EF-G to the ribosome (Bodley et al., 1970; Hausner et al., 1987; Cameron et al., 2002), suggesting that Tet(O) and EF-G depend on the same ribosomal elements for binding. However, the specifics of this interaction are clearly different, as illustrated in Figure 5A and B where the sites of interaction in H42/43/44 differ between EF-G and Tet(O) (green squares and red circles, respectively). The unique interaction of Tet(O) is probably dictated by the fact that the L11 region is proposed to undergo conformational changes during elongation (Wimberly et al., 1999) and, therefore, since Tet(O) interacts at a step different from EF-G, it must recognize an altered conformation.
Fig. 5. (A and B) Summary of the changes in DMS modification in the L11 region (A) and the α-sarcin loop (B). The positions whose DMS reactivity changes in response to EF-G (green squares) or Tet(O) (red circles) binding are marked on the secondary structure (Cannone et al., 2002) of the L11 region (H42/43/44; nucleotides 1036–1119) and the α-sarcin loop (H95; nucleotides 2647–2673). The protection of A1069 by Tet(O) is weak but is included because it was observed consistently in several reverse transcriptions experiments. (C) The locations of the Tet(O)- and EF-G-associated protections are shown on the crystal structure of the L11 region. The protections are modeled on the structure of the L11 region (Protein Data Bank accession code: 1MMS; Wimberly et al., 1999). The 23S rRNA of the L11 region (1051–1108) is shown as a gray ribbon, and the protein L11 is colored cyan. A1070, which is concealed and protected by Tet(O), is colored red, while the exposed A1067 is protected by EF-G and colored green. The structure was illustrated using Ribbons (Carson, 1991).
When the sites that experience changes in DMS reactivity upon EF-G or Tet(O) binding are viewed on the three-dimensional structure (Wimberly et al., 1999) of the L11 region as in Figure 5C, A1067:N1 (protected by EF-G; Figure 5C, green wire frame) seems to be exposed to the elongation factor, whereas A1070:N1 [protected by Tet(O); Figure 5C, red wire frame] is less accessible. The change in the chemical accessibility of A1070, being in a confined location, could be direct where a conformational change in the L11 or the rRNA allows Tet(O) access to this base, or indirect where a change in L11 or the rRNA leads to the protection of this base. In any case, a conformational change is involved, and evidence of this conformational change is also illustrated by the enhanced DMS modification of A1050 upon Tet(O)·GMPPNP binding, which could reflect the movement of the L11 region around a ‘hinge’ that brings it closer to the SRL (Gabashvili et al., 2000). Interestingly, cryo-EM models (Spahn et al., 2001) indicate that domain V in Tet(O) is closer to the N-terminal domain of L11 compared with EF-G, possibly leading to the difference in their interaction.
Further evidence for a Tet(O)-dependent change in the GAR of the 50S subunit derives from the fact that Tet(O) is able to stimulate the ribosome-dependent GTPase activity of EF-Tu (Figure 2C). Cryo-EM reconstructions (Stark et al., 1997; Spahn et al., 2001; Valle et al., 2002) and chemical probing experiments (Moazed et al., 1988; this study) show that EF-Tu and Tet(O) occupy overlapping sites on the ribosome, and therefore can not occupy the ribosome simultaneously. In this respect, the stimulation of EF-Tu’s GTPase activity is indicative that Tet(O) stabilizes a conformation of the GAR such that upon interaction with a population of empty ribosomes, it leaves the ribosomes in a configuration that is favorable for the interaction of EF-Tu. This feature would be important in vivo because, after Tet(O) removes tetracycline, the ternary complex (EF-Tu·GTP·aa-tRNA) must bind efficiently for protein synthesis to continue.
It follows that Tet(O) may also enhance the ability of the ternary complex to compete with tetracycline for the A-site, by invoking conformational changes that disrupt the subsequent interaction of tetracycline with the A-site. This interpretation is supported by two lines of evidence: (i) Tet(O) chases tetracycline by disrupting the conformation of its binding site (Spahn et al., 2001); and (ii) the enhancement of A1408 in h44 to DMS modification by Tet(O), which is an indication of rearrangements in the decoding site (Connell et al., 2002), remains after Tet(O) leaves the ribosome since this enhancement is also observed when GTP is used instead of a non-hydrolyzable GTP analog (S.R.Connell, unpublished data).
Additionally, biochemical evidence exists to suggest that tetracycline also induces structural changes in the ribosome (Dahlberg et al., 1973; Tritton, 1977; Noah et al., 1999). DMS probing experiments support at least a local conformational change, since the modification of bases U1052 and C1054 of 16S rRNA were enhanced in the presence of the drug (Moazed and Noller, 1987; Connell et al., 2002), and a change in the position of C1054 is even visualized by X-ray crystallography (Brodersen et al., 2000; Pioletti et al., 2001). In this sense, the activation energy associated with tetracycline binding (Ea = 58 kJ/mol) could be involved in promoting these changes. In fact, activation energies of the magnitude derived for tetracycline binding often ‘involve entropy effects due to structural rearrangements’ in biological systems, although protein gross conformational changes characteristically have larger values of 90–120 kJ/mol (Gutfreund, 1995).
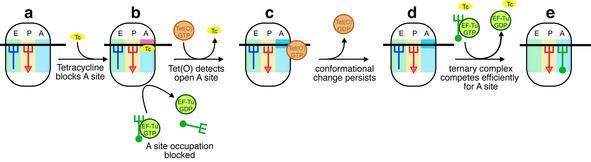
Figure 6 shows a model derived from current and previous studies on RPPs. Initially (step a) tetracycline binds to the POST state ribosome, induces a conformational change (Ea = 58 kJ/mol) without releasing the E-site-bound tRNA, and blocks the ternary complex from occupying the A-site (step b). Tet(O), which is present in low abundance within the cell, recognizes this blocked ribosome by virtue of its open A-site, prolonged pausing in the POST state and possibly by the drug-induced conformational change. The interaction of Tet(O) with the ribosome triggers the release of tetracycline prior to GTP hydrolysis (Trieber et al., 1998) and induces rearrangements in the A-site, as evidenced by changes in the DMS reactivity of A1408 of the 16S rRNA (Connell et al., 2002; step c). Tet(O) then hydrolyzes the bound GTP and leaves the ribosome with the elongation factor-binding region in a configuration compatible with EF-Tu binding. A shift in the conformation of this region is supported by the change in DMS reactivity of A1070 upon Tet(O) binding to the ribosome and the stimulation of the GTPase activity of EF-Tu. The latter effect suggests that the conformational changes in the A-site remain after Tet(O) release (step d), enhancing the ability of ternary complex to compete with tetracycline in the subsequent round of A-site occupation (step e). This model presents the mechanism of Tet(O)-mediated resistance in the context of the overall elongation cycle such that further experimentation may now be carried out to validate the kinetic aspects of Tet(O) action.
Fig. 6. A model for Tet(O)-mediated tetracycline resistance. The binding of tetracycline to the elongating ribosome (a) is accompanied by a conformational change in the decoding site, but not the release of the E-site-bound tRNA (b; indicated by the blue to red color change). The binding of tetracycline blocks subsequent EF-Tu·GTP·aa-tRNA-dependent occupation of the A-site by sterically interfering with the accommodation of the aa-tRNA (Brodersen et al., 2000; Pioletti et al., 2001). Tet(O) binds to the tetracycline-blocked ribosome (c) and, in doing so, triggers the release of the bound tetracycline by changing the conformation of the decoding site or, more specifically, h34 and h44 (Spahn et al., 2001; Connell et al., 2002). Next, the GTPase activity of Tet(O) is activated and it is released from the ribosome, leaving the decoding site in a conformation (d; indicated with dark blue) that disfavors tetracycline binding, allowing ternary complex to compete efficiently for the A-site (e).
Materials and methods
Purified histidine-tagged EF-G and EF-Tu from E.coli were provided by Ulrich Stelzl. The AcPhe-tRNAPhe was prepared as described previously (Rheinberger et al., 1988), and the MF-mRNA was prepared by Detlev Kamp. All other materials were purchased from commercial suppliers.
Construction and purification of Tet(O)HN
tet(O) was amplified from pUAO2E1 (Wang and Taylor, 1991) and cloned as previously described (Trieber et al., 1998), except that the primers used encoded a His6 tag at the 5′ end of tetO. N-terminally His-tagged Tet(O) [Tet(O)HN] was purified as previously described (Trieber et al., 1998) with the following modifications: (i) the Ni2+ column (chelating Sepharose; Pharmacia) was washed with 50 mM imidazole and eluted with 300 mM imidazole and (ii) the buffer was exchanged after the Ni2+ column for 50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 10% glycerol and 0.5 mM dithiothretol (DTT) using a G-75 Sephadex column (Pharmacia).
Construction and purification of Tet(O)D131N
tet(O) was amplified from pUOA2E1 (Wang and Taylor, 1991) by PCR as previously described (Trieber et al., 1998) and cloned into the phagemid pTZ19R (Mead et al., 1986). Using the Sculptor in vitro mutagenesis system (Amersham Biosciences), Asp131 was changed to asparagine. The mutation was confirmed by sequencing, and a 0.7 kb EcoRI–Eco47III fragment of the tet(O) gene containing the mutation was subcloned into pMS119-Tet(O)-H (Trieber et al., 1998), yielding pMS119-Tet(O)D131N. The protein was purified as described previously (Trieber et al., 1998).
Preparation of defined ribosomal complexes
Pre- and post-translocational complexes used in Figure 1B–D were made as described previously (Blaha et al., 2000). The PRE complex consisted of re-associated 70S ribosomes (Blaha et al., 2000) programmed with MF-mRNA, a deacyl-tRNAfMet in the P-site and Ac[14C]Phe-tRNAPhe in the A-site, and subsequently were translocated by EF-G to yield the POST complexes. The PRE and POST complexes were then sedimented through a 10% sucrose cushion in a TLA 100.3 rotor (RCFavg = 76 000, 17 h, 4°C) to remove EF-G and exchange the buffer for H20M10N100SH4 (20 mM HEPES–KOH pH 7.5, 10 mM Mg acetate, 100 mM NH4 acetate and 4 mM β-mercaptoethanol). The homogeneity of the complex can be illustrated by the ratio of the puromycin-reactive tRNA in the PRE state to that in the POST state (PM+pre/PM+post; Polacek et al., 2000). These PRE and POST complexes contained 0.7 AcPhe-tRNAPhe per 70S, and the homogeneity of the complex was 97% as determined by the puromycin reactivity.
PRE and POST complexes used in Figure 1E were constructed as described above; however, they employed tight-coupled 70S ribosomes (Bommer et al., 1996) rather than re-associated 70S ribosomes, and as such had lower tRNA binding (0.3 AcPhe-tRNAPhe per 70S ribosome, with 97% homogeneity) and were maintained in H20M6N150SH4Spd2Sp0.05.
For the DMS probing experiments in Figure 3A, AcPhe-tRNAPhe was bound to the P-site of tight-coupled 70S ribosomes in the presence of poly(U) mRNA (0.7 AcPhe-tRNAPhe per 70S ribosome) and maintained in H20M6N150SH4Spd2Sp0.05 buffer (Blaha et al., 2000).
For E-site binding in the presence of tetracycline, the POST complex was prepared such that 0.62 µM [32P]tRNAfMet and 0.46 µM Ac[14C]Phe-tRNAPhe were bound to MF-mRNA-programmed 70S ribosomes (0.31 µM) in the presence of EF-G·GTP under H20M6N150SH4Spd2Sp0.05 buffer conditions. Where indicated, tetracycline was present at 370 µM. tRNA binding was monitored in nitrocellulose filter binding assays and puromycin reactivity assays (Blaha et al., 2000).
DMS modification of Tet(O)·ribosomal complexes
Tet(O) was bound to the ribosomal complexes by incubating 100 pmol of purified Tet(O) with 2.5 nmol of GMPPNP and 25 pmol of the desired ribosomal complex in 50 µl of H20M10N100SH4 buffer (experiments described in Figure 1) or H20M6N150SH4Spd2Sp0.05 buffer (experiments described in Figure 3) for 15 min at 37°C. Subsequently, the complexes were chemically modified, analyzed by primer extension analysis, and quantified as described previously (Connell et al., 2002). The values reported in the text for changes in DMS reactivity correspond to the ratio of the intensity of a band corresponding to a DMS-dependent stop in a sample lane [i.e. Tet(O) + 70S ribosomes] compared with that in a control lane (i.e. 70S ribosomes).
Isolation of EF-Tu·GTP complex
GTP was exchanged for GDP bound to EF-Tu as previously described (Mesters et al., 1994), with some modifications. Briefly, this reaction is carried out in H20M6N150SH4Spd2Sp0.05 buffer with 50 µM [γ-32P]GTP, 1 mM phosphoenolpyruvate (PEP), 0.1 mg/ml pyruvate kinase and 2 µM EF-Tu. This reaction is incubated at 37°C for 10 min. Next, the EF-Tu·GTP complex was isolated by loading the binding reaction on a G-50 spun column (Boehringer Mannheim) and centrifuged for 2 min at 1100 g. The EF-Tu·GTP complex is collected in the first fraction.
GTPase activity
The GTPase assays were as described previously (Dasmahapatra and Chakraburtty, 1981) except that reactions were set up to maintain H20M6N150SH4Spd2Sp0.05 buffer conditions with a final ribosome concentration of 0.2 µM, a final protein concentration of 0.2 µM and a nucleotide (XTP or [γ-32P]GTP) concentration of 50 µM.
Tetracycline binding
Tetracycline binding was measured using [3H]tetracycline in a nitrocellulose binding assay (Trieber et al., 1998). The activation energy of the binding reaction was determined as described (Schilling-Bartetzko et al., 1992) using the Arrhenius equation. This involved the underlying assumption that the rate-limiting step of tetracycline binding follows pseudo-first order reaction kinetics such that:
where dpmmax is the maximal amount of [3H]tetracycline (measured in d.p.m.) retained on the filter, and dpmt is amount of [3H]tetracycline (measured in d.p.m.) retained on the filter at time t. The apparent rate constants, k, are then derived from this plot and their temperature dependence determined according to the Arrhenius equation:
where A is the frequency factor, Ea is the activation energy, R is the universal gas constant [8.314 J/(mol·K)]) and T is the absolute temperature. The slope of a line resulting from a plot of ln(k) versus 1/T will be equal to the (Ea/R).
Acknowledgments
Acknowledgements
We would like to extend our thanks to P.M.Fucini, D.Wilson, N.Polacek and U.Stelzl for support and helpful discussions. This work was funded by the Alberta Heritage Foundation through an AHFMR Studentship to S.R.C. and an AHFMR Scientist Award to D.E.T., a grant from the National Science and Engineering Research Council of Canada (NSERC) to D.E.T., and a grant from the Deutsche Forschungsgemeinschaft to K.H.N. (Ni174/8-3).
References
- Blaha G., Stelzl,U., Spahn,C.M., Agrawal,R.K., Frank,J. and Nierhaus,K.H. (2000) Preparation of functional ribosomal complexes and effect of buffer conditions on tRNA positions observed by cryoelectron microscopy. Methods Enzymol., 317, 292–309. [DOI] [PubMed] [Google Scholar]
- Bodley J.W., Lin,L. and Highland,J.H. (1970) Studies on translocation. VI. Thiostrepton prevents the formation of a ribosome–G factor–guanine nucleotide complex. Biochem. Biophys. Res. Commun., 41, 1406–1411. [DOI] [PubMed] [Google Scholar]
- Bommer U., Burkhardt,N., Jünemann,R., Spahn,C.M.T., Triana-Alonso,F.J. and Nierhaus,K.H. (1996) Ribosomes and polysomes. In Graham,J. and Rickwood,D. (eds), Subcellular Fractionation. A Practical Approach. IRL Press at Oxford University Press, Oxford, UK, pp. 271–301.
- Brodersen D.E., Clemons,W.M., Carter,A.P., Morgan-Warren,R.J., Wimberly,B.T. and Ramakrishnan,V. (2000) The structural basis for the action of the antibiotics tetracycline, pactamycin and hygromycin B on the 30S ribosomal subunit. Cell, 103, 1143–1154. [DOI] [PubMed] [Google Scholar]
- Burdett V. (1991) Purification and characterization of Tet(M), a protein that renders ribosomes resistant to tetracycline. J. Biol. Chem., 266, 2872–2877. [PubMed] [Google Scholar]
- Burdett V. (1996) Tet(M)-promoted release of tetracycline from ribosomes is GTP dependent. J. Bacteriol., 178, 3246–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D.M., Thompson,J., March,P.E. and Dahlberg,A.E. (2002) Initiation factor IF2, thiostrepton and micrococcin prevent the binding of elongation factor G to the Escherichia coli ribosome. J. Mol. Biol., 319, 27–35. [DOI] [PubMed] [Google Scholar]
- Cannone J.J. et al. (2002) The Comparative RNA Web (CRW) Site: an online database of comparative sequence and structure information for ribosomal, intron and other RNAs. BMC Bioinformatics, 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M. (1991) Ribbons 2.0. J. Appl. Crystallogr., 24, 103–106. [Google Scholar]
- Chopra I. and Roberts,M. (2001) Tetracycline antibiotics: mode of action, applications, molecular biology and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev., 65, 232–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell S.R., Trieber,C.A., Stelzl,U., Einfeldt,E., Taylor,D.E. and Nierhaus,K.H. (2002) The tetracycline resistance protein, Tet(O), perturbs the conformation of the ribosomal decoding center. Mol. Microbiol., 45, 1463–1472. [DOI] [PubMed] [Google Scholar]
- Dahlberg A.E., Lund,E., Kjeldgaard,N.O., Bowman,C.M. and Nomura,M. (1973) Colicin E3 induced cleavage of 16S ribosomal ribonucleic acid; blocking effects of certain antibiotics. Biochemistry, 12, 948–950. [DOI] [PubMed] [Google Scholar]
- Dantley K.A., Dannelly,H.K. and Burdett,V. (1998) Binding interaction between Tet(M) and the ribosome: requirements for binding. J. Bacteriol., 180, 4089–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra B. and Chakraburtty,K. (1981) Protein synthesis in yeast. I. Purification and properties of elongation factor 3 from Saccharomyces cerevisiae. J. Biol. Chem., 256, 9999–10004. [PubMed] [Google Scholar]
- Endo Y. and Wool,I.G. (1982) The site of action of α-sarcin on eukaryotic ribosomes. The sequence at the α-sarcin cleavage site in 28S rRNA. J. Biol. Chem., 257, 9054–9060. [PubMed] [Google Scholar]
- Fernandez-Puentes C. and Vazquez,D. (1977) Effects of some proteins that inactivate the eukaryotic ribosome. FEBS Lett., 78, 143–146. [DOI] [PubMed] [Google Scholar]
- Gabashvili I.S., Agrawal,R.K., Spahn,C.M., Grassucci,R.A., Svergun,D.I., Frank,J. and Penczek,P. (2000) Solution structure of the E.coli 70S ribosome at 11.5 Å resolution. Cell, 100, 537–549. [DOI] [PubMed] [Google Scholar]
- Grewal J., Manavathu,E.K. and Taylor,D.E. (1993) Effect of mutational alteration of Asn-128 in the putative GTP-binding domain of tetracycline resistance determinant Tet(O) from Campylobacter jejuni. Antimicrob. Agents Chemother., 37, 2645–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutfreund H. (1995) Kinetics for the Life Sciences: Receptors, Transmitters and Catalysts. Cambridge University Press.
- Hausner T.P., Atmadja,J. and Nierhaus,K.H. (1987) Evidence that the G2661 region of 23S rRNA is located at the ribosomal binding sites of both elongation factors. Biochimie, 69, 911–923. [DOI] [PubMed] [Google Scholar]
- Hwang Y.W. and Miller,D.L. (1987) A mutation that alters the nucleotide specificity of elongation factor Tu, a GTP regulatory protein. J. Biol. Chem., 262, 13081–13085. [PubMed] [Google Scholar]
- Leipe D.D., Wolf,Y.I., Koonin,E.V. and Aravind,L. (2002) Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol., 317, 41–72. [DOI] [PubMed] [Google Scholar]
- Manavathu E.K., Fernandez,C.L., Cooperman,B.S. and Taylor,D.E. (1990) Molecular studies on the mechanism of tetracycline resistance mediated by Tet(O). Antimicrob. Agents Chemother., 34, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D.A., Szczesna-Skorupa,E. and Kemper,B. (1986) Single-stranded DNA ‘blue’ T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng., 1, 67–74. [DOI] [PubMed] [Google Scholar]
- Mesters J.R., Potapov,A.P., Degraaf,J.M. and Kraal,B. (1994) Synergism between the GTPase activities of EF-Tu·GTP and EF-G·GTP on empty ribosomes—elongation factors as stimulators of the ribosomal oscillation between two conformations. J. Mol. Biol., 242, 644–654. [DOI] [PubMed] [Google Scholar]
- Moazed D. and Noller,H.F. (1987) Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature, 327, 389–394. [DOI] [PubMed] [Google Scholar]
- Moazed D., Robertson,J.M. and Noller,H.F. (1988) Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature, 334, 362–364. [DOI] [PubMed] [Google Scholar]
- Nierhaus K.H., Schilling-Bartetzko,S. and Twardowski,T. (1992) The two main states of the elongating ribosome and the role of the α-sarcin stem–loop structure of 23S RNA. Biochimie, 74, 403–410. [DOI] [PubMed] [Google Scholar]
- Noah J.W., Dolan,M.A., Babin,P. and Wollenzien,P. (1999) Effects of tetracycline on the tertiary structure of ribosomal RNA in the Escherichia coli 30S ribosomal subunit. J. Biol. Chem., 274, 16576–16581. [DOI] [PubMed] [Google Scholar]
- Pestka S. (1970) Thiostrepton: a ribosomal inhibitor of translocation. Biochem. Biophys. Res. Commun., 40, 667–674. [DOI] [PubMed] [Google Scholar]
- Pioletti M. et al. (2001) Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J., 20, 1829–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacek N., Patzke,S., Nierhaus,K.H. and Barta,A. (2000) Periodic conformational changes in rRNA: monitoring the dynamics of translating ribosomes. Mol. Cell, 6, 159–171. [PubMed] [Google Scholar]
- Porse B.T., Leviev,I., Mankin,A.S. and Garrett,R.A. (1998) The antibiotic thiostrepton inhibits a functional transition within protein L11 at the ribosomal GTPase centre. J. Mol. Biol., 276, 391–404. [DOI] [PubMed] [Google Scholar]
- Rheinberger H.J. and Nierhaus,K.H. (1986) Allosteric interactions between the ribosomal transfer RNA-binding sites A and E. J. Biol. Chem., 261, 9133–9139. [PubMed] [Google Scholar]
- Rheinberger H.J., Geigenmüller,U., Wedde,M. and Nierhaus,K.H. (1988) Parameters for the preparation of Escherichia coli ribosomes and ribosomal subunits active in tRNA binding. Methods Enzymol., 164, 658–670. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Brown,J.T., Roberts,M. and Urdea,M.S. (1988) Homology of the TetM with translational elongation factors: implications for potential modes of tetM-conferred tetracycline resistance. Nucleic Acids Res., 16, 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling-Bartetzko S., Bartetzko,A. and Nierhaus,K.H. (1992) Kinetic and thermodynamic parameters for tRNA binding to the ribosome and for the translocation reaction. J. Biol. Chem., 267, 4703–4712. [PubMed] [Google Scholar]
- Spahn C.M.T. et al. (2001) Localization of the ribosomal protection protein Tet(O) on the ribosome and the mechanism of tetracycline resistance. Mol. Cell, 7, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Stark H., Rodnina,M.V., Rinke-Appel,J., Brimacombe,R., Wintermeyer, W. and van Heel,M. (1997) Visualization of elongation factor Tu on the Escherichia coli ribosome. Nature, 389, 403–406. [DOI] [PubMed] [Google Scholar]
- Stern S., Moazed,D. and Noller,H.F. (1988) Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol., 164, 481–489. [DOI] [PubMed] [Google Scholar]
- Trieber C.A., Burkhardt,N., Nierhaus,K.H. and Taylor,D.E. (1998) Ribosomal protection from tetracycline mediated by Tet(O): Tet(O) interaction with ribosomes is GTP-dependent. Biol. Chem., 379, 847–855. [DOI] [PubMed] [Google Scholar]
- Tritton T.R. (1977) Ribosome–tetracycline interactions. Biochemistry, 16, 4133–4138. [DOI] [PubMed] [Google Scholar]
- Valle M., Sengupta,J., Swami,N.K., Grassucci,R.A., Burkhardt,N., Nierhaus,K.H., Agrawal,R.K. and Frank,J. (2002) Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. EMBO J., 21, 3557–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. and Taylor,D.E. (1991) A DNA sequence upstream of the tet(O) gene is required for full expression of tetracycline resistance. Antimicrob. Agents Chemother., 35, 2020–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijland A. and Parmeggiani,A. (1993) Toward a model for the interaction between elongation factor Tu and the ribosome. Science, 259, 1311–1314. [DOI] [PubMed] [Google Scholar]
- Weijland A., Parlato,G. and Parmeggiani,A. (1994) Elongation factor Tu D138N, a mutant with modified substrate specificity, as a tool to study energy consumption in protein biosynthesis. Biochemistry, 33, 10711–10717. [DOI] [PubMed] [Google Scholar]
- Wimberly B.T., Guymon,R., McCutcheon,J.P., White,S.W. and Ramakrishnan,V. (1999) A detailed view of a ribosomal active site: the structure of the L11–RNA complex. Cell, 97, 491–502. [DOI] [PubMed] [Google Scholar]
- Wool I.G., Gluck,A. and Endo,Y. (1992) Ribotoxin recognition of ribosomal RNA and a proposal for the mechanism of translocation. Trends Biochem. Sci., 17, 266–269. [DOI] [PubMed] [Google Scholar]