Abstract
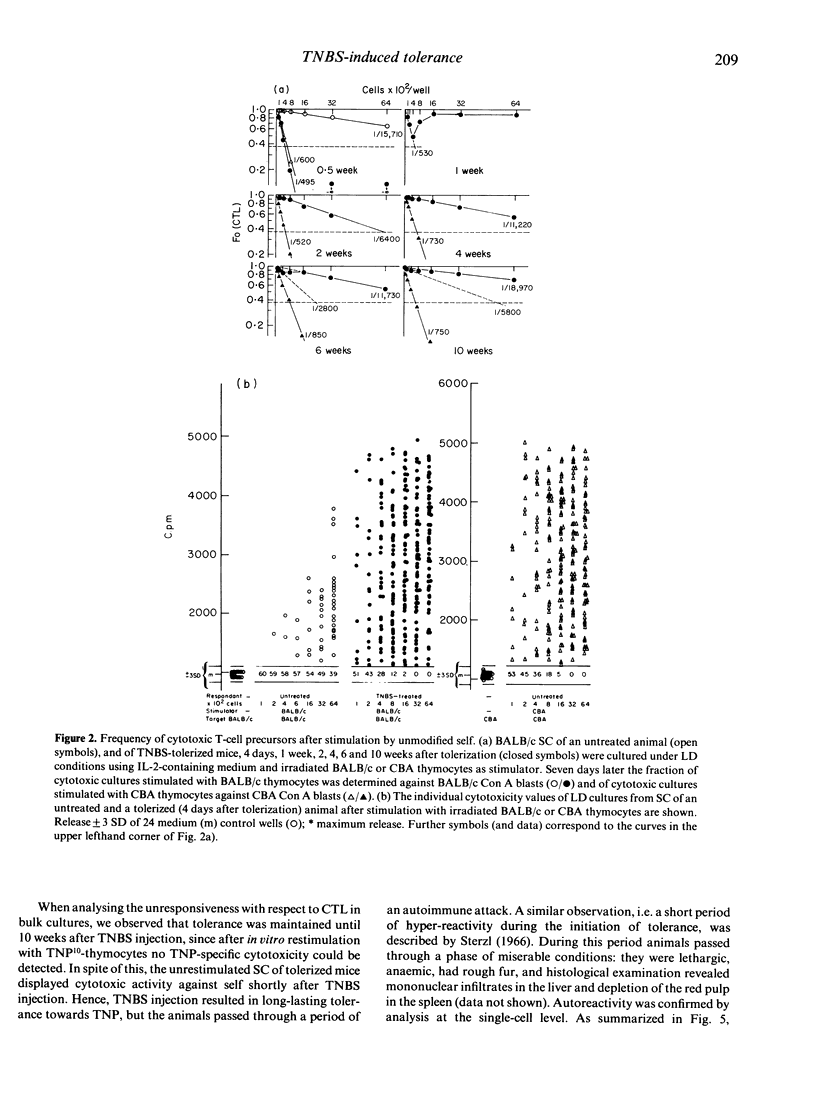
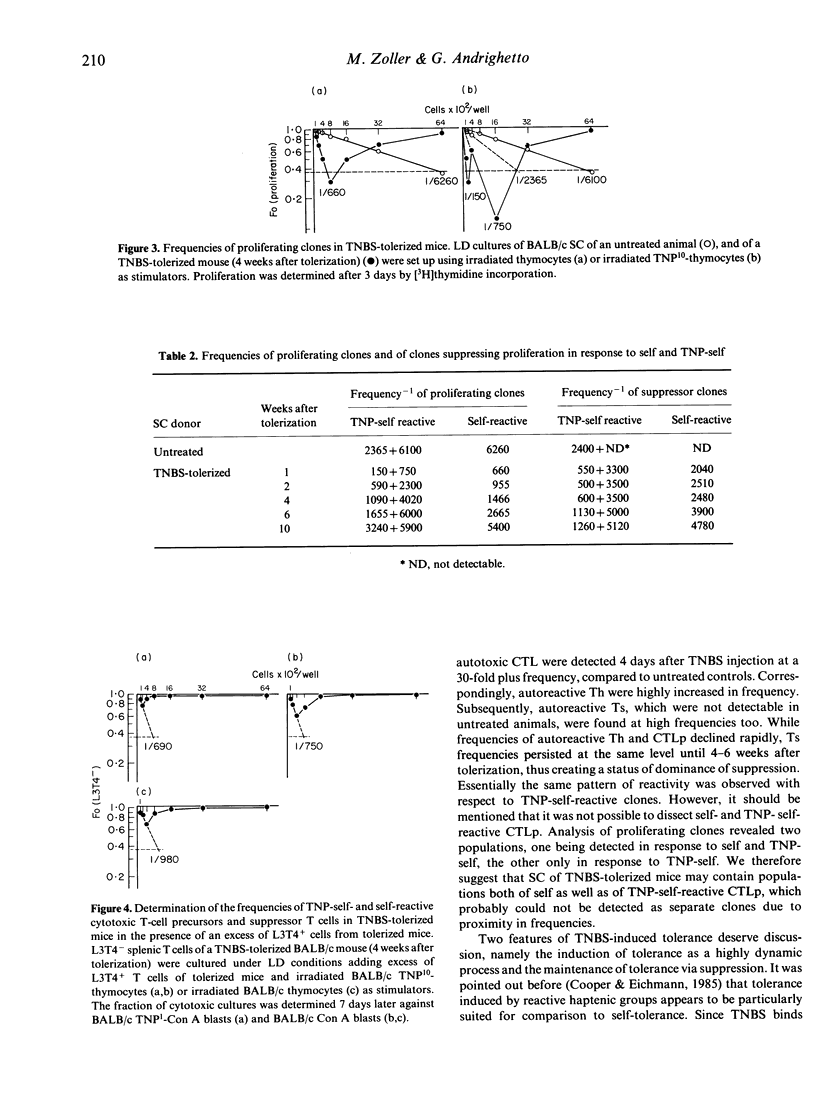
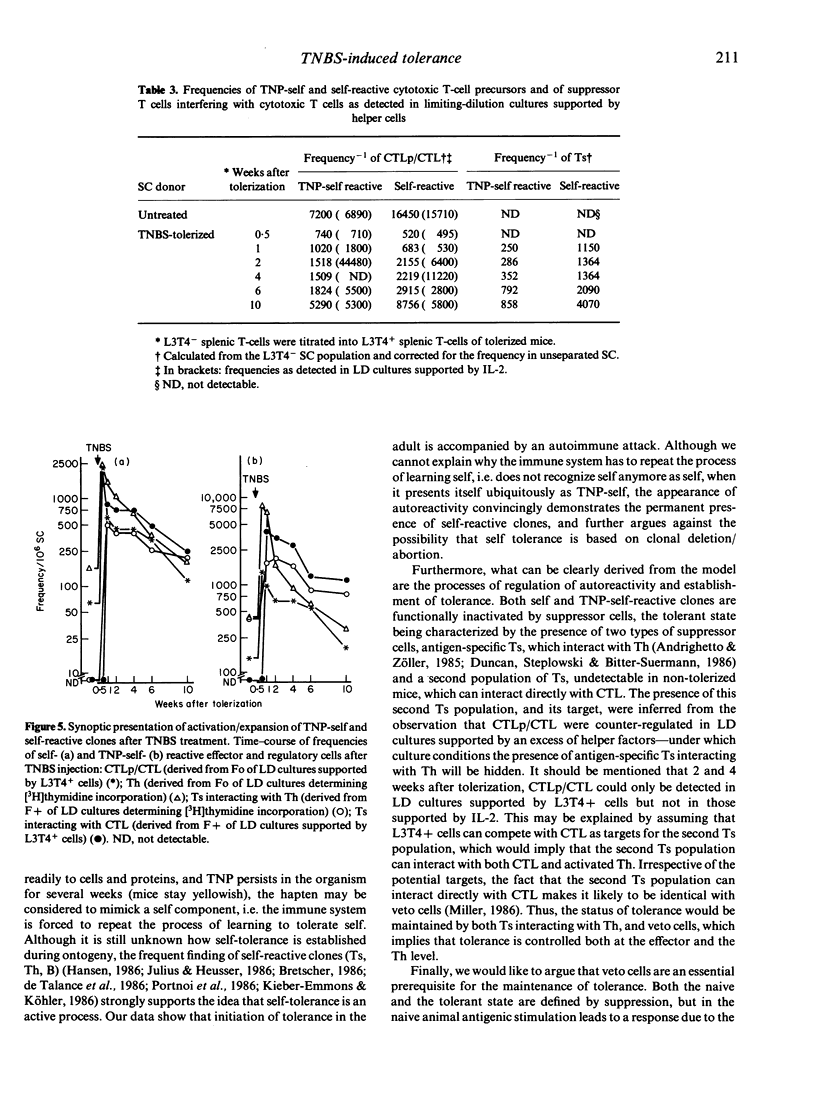
In order to follow the process of induction and maintainance of tolerance, BALB/c mice were tolerized by free hapten, and effector and regulatory cell interactions were analysed by limiting-dilution (LD) cultures. Injection of trinitrobenzenesulphonic acid (TNBS) resulted, predominantly, in the activation and expansion of self-reactive cytotoxic T cells (CTL), which were observed transiently at frequencies comparable to allo-specific CTL. In addition, self-reactive helper T cells (Th) were activated and expanded in tolerized mice. TNP-specific reactivity was difficult to evaluate, since cytotoxic activity against haptenized self followed the pattern of self-reactivity throughout the test period. But in LD cultures determining proliferation, two populations of Th responding to TNP-self were observed, while only one Th population could be detected in response to self. Expansion/activation of Th and CTL precursors (CTLp) was followed by activation of suppressor T cells (Ts). The suppressor population could be divided into two subpopulations, one interfering with Th, the second interacting directly with CTL (veto cells). The results indicate that during the induction of tolerance, animals pass through an autoimmune attack, with expansion and activation of self-reactive clones (CTL, Th). The final status of non-responsiveness towards TNP is not due to the deletion of effector or regulatory cells, but results from the establishment of a steady state of dominance of self-reactive and TNP-self-reactive suppression.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrighetto G., Zöller M. Time course analysis of regulation of cytotoxic T cells. Cell Immunol. 1985 Jun;93(1):178–188. doi: 10.1016/0008-8749(85)90398-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. M., Eichmann K. Limiting dilution analysis of induced unresponsiveness to trinitrophenyl: increased suppression of cytotoxic T precursor cells at unchanged frequencies. J Mol Cell Immunol. 1985;2(2):83–94. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Duncan W. R., Stepkowski S. M., Bitter-Suermann H. Induction of transplantation tolerance in rats by spleen allografts. I. Evidence that rats tolerant of spleen allografts contain two phenotypically distinct T suppressor cells. Transplantation. 1986 May;41(5):626–633. doi: 10.1097/00007890-198605000-00015. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Fey K., Kuppers R., Melchers I., Simon M. M., Weltzien H. U. Network regulation among T cells; conclusions from limiting dilution experiments. Springer Semin Immunopathol. 1983;6(1):7–32. doi: 10.1007/BF01857364. [DOI] [PubMed] [Google Scholar]
- Fidler J. M., Golub E. S. Immunological tolerance to a hapten. I. Induction and maintenance of tolerance to trinitrophenyl with trinitrobenzene sulfonic acid. J Exp Med. 1973 Jan 1;137(1):42–54. doi: 10.1084/jem.137.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby P. A., Kotzin B. L., Kansas G. S., Engleman E. G. Immunoglobulin secretion in the human autologous mixed leukocyte reaction. Definition of a suppressor-amplifier circuit using monoclonal antibodies. J Exp Med. 1982 Jul 1;156(1):55–67. doi: 10.1084/jem.156.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J., Basten A., Walker K. Z., Loblay R. H. A role for suppressor T cells in induction of self-tolerance. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5150–5154. doi: 10.1073/pnas.82.15.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B. L. Why do some individuals produce autoreactive antibodies against receptors and/or their ligands? A possible answer to the question. A review with implications. Scand J Immunol. 1986 Oct;24(4):363–370. doi: 10.1111/j.1365-3083.1986.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Heusser C. H. Autoreactive or self restricted? Eur J Immunol. 1986 Jul;16(7):867–870. doi: 10.1002/eji.1830160726. [DOI] [PubMed] [Google Scholar]
- Kieber-Emmons T., Kohler H. Evolutionary origin of autoreactive determinants (autogens). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2521–2525. doi: 10.1073/pnas.83.8.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkovits L., Aarden L. A., Corley R. B. Ratio-dominance model of suppression: an analysis by limiting dilution. Immunology. 1980 Oct;41(2):407–413. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Oki A., Sercarz E. T cell tolerance studied at the level of antigenic determinants. I. Latent reactivity to lysozyme peptides that lack suppressogenic epitopes can be revealed in lysozyme-tolerant mice. J Exp Med. 1985 May 1;161(5):897–911. doi: 10.1084/jem.161.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoï D., Freitas A., Holmberg D., Bandeira A., Coutinho A. Immunocompetent autoreactive B lymphocytes are activated cycling cells in normal mice. J Exp Med. 1986 Jul 1;164(1):25–35. doi: 10.1084/jem.164.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammensee H. G., Bevan M. J. Evidence from in vitro studies that tolerance to self antigens is MHC-restricted. Nature. 1984 Apr 19;308(5961):741–744. doi: 10.1038/308741a0. [DOI] [PubMed] [Google Scholar]
- Smart Y. C., Cox J., Roberts T. K., Brinsmead M. W., Burton R. C. Differential effect of cigarette smoking on recirculating T lymphocyte subsets in pregnant women. J Immunol. 1986 Jul 1;137(1):1–3. [PubMed] [Google Scholar]
- Sterzl J. Immunological tolerance as the result of terminal differentiation of immunologically competent cells. Nature. 1966 Jan 22;209(5021):416–417. doi: 10.1038/209416a0. [DOI] [PubMed] [Google Scholar]
- Stockinger B. Cytotoxic T-cell precursors revealed in neonatally tolerant mice. Proc Natl Acad Sci U S A. 1984 Jan;81(1):220–223. doi: 10.1073/pnas.81.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. J., Strome P. G., Streilein J. W. Clonal analysis of helper and effector T-cell function in neonatal transplantation tolerance: clonal deletion of helper cells determines lack of in vitro responsiveness. Immunogenetics. 1984;20(2):185–196. doi: 10.1007/BF00364489. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauderer M., Campbell H., Johnson D. R., Seman M. Helper functions of antigen-induced specific and autoreactive T cell colonies. J Mol Cell Immunol. 1984;1(2):65–77. [PubMed] [Google Scholar]
- Zöller M., Andrighetto G. Comparison between the in vitro activities of in vivo-induced hapten-specific suppressor cells and supernatants of a hapten-specific suppressor hybridoma. Cell Immunol. 1984 Dec;89(2):310–321. doi: 10.1016/0008-8749(84)90333-2. [DOI] [PubMed] [Google Scholar]
- Zöller M., Andrighetto G. Limiting dilution analysis of the frequencies of helper and suppressor T cells in untreated and TNP-treated BALB/c mice: response as a consequence of perturbation of a stable steady state. Immunology. 1985 Aug;55(4):703–712. [PMC free article] [PubMed] [Google Scholar]
- Zöller M., Lopatta D., Benato B., Andrighetto G. Oscillation of antibody production and regulatory T cells in response to antigenic stimulation. Eur J Immunol. 1985 Dec;15(12):1198–1203. doi: 10.1002/eji.1830151211. [DOI] [PubMed] [Google Scholar]



