Abstract
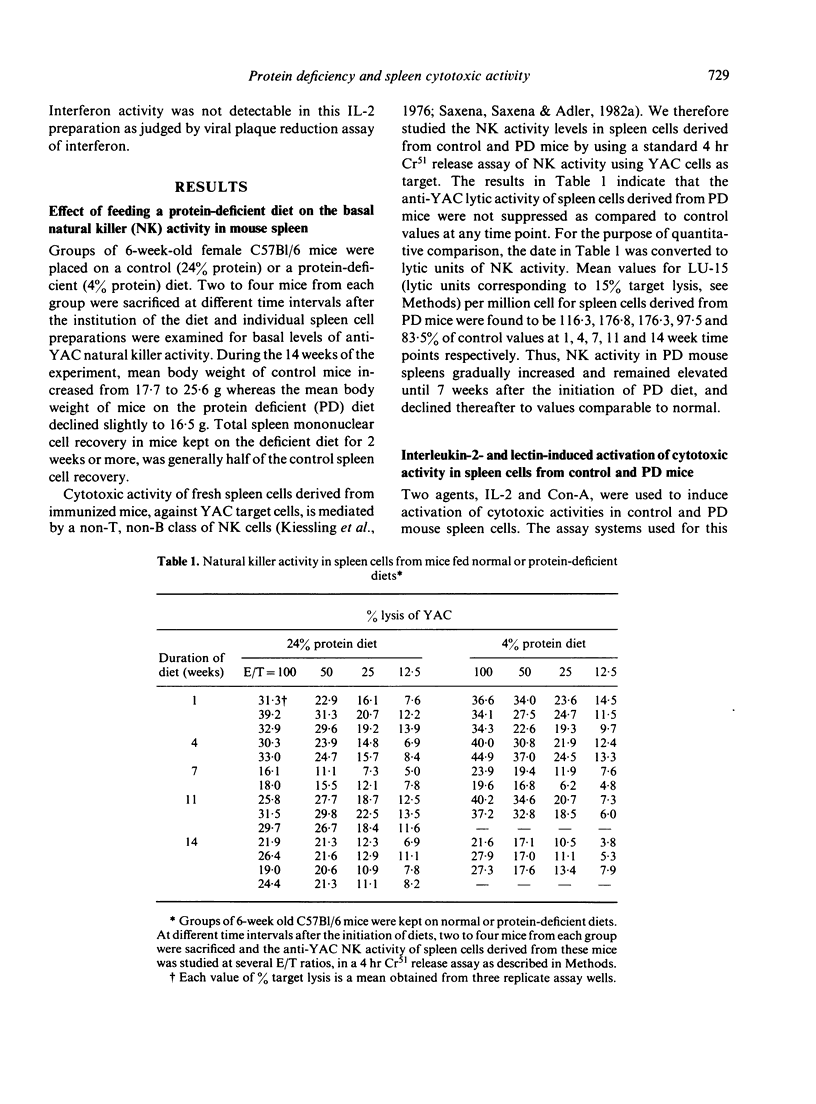
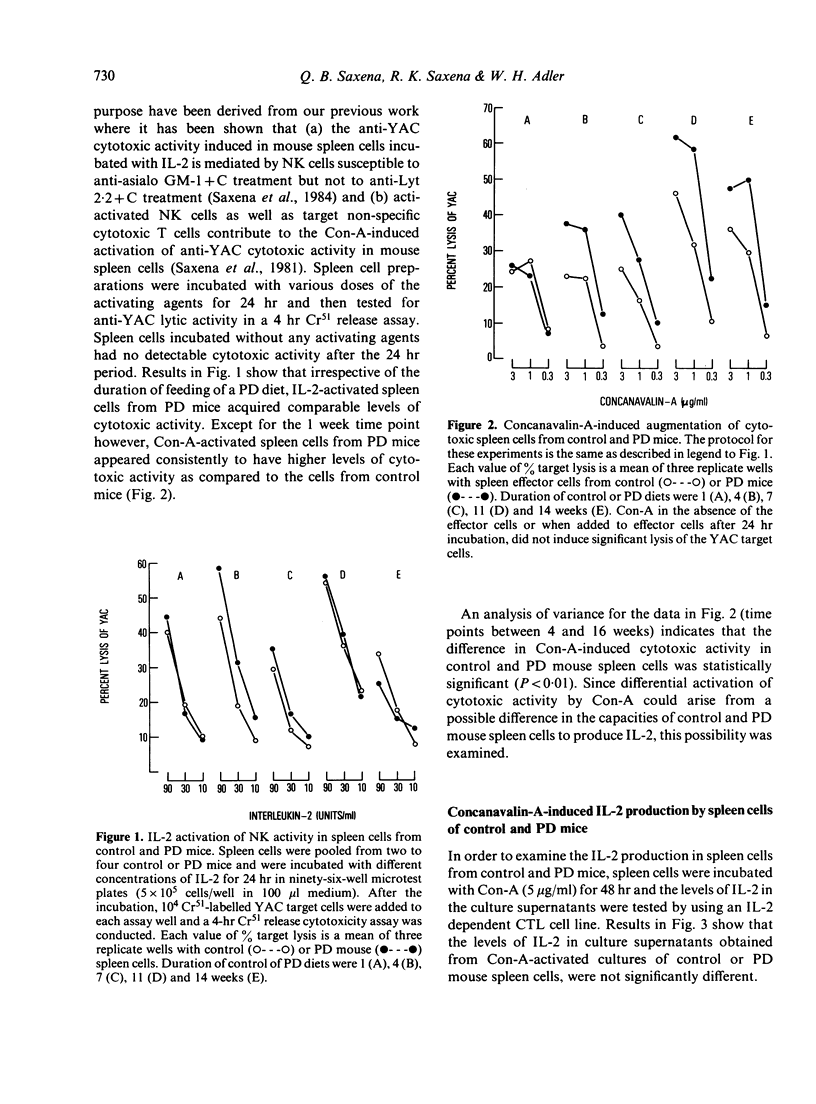
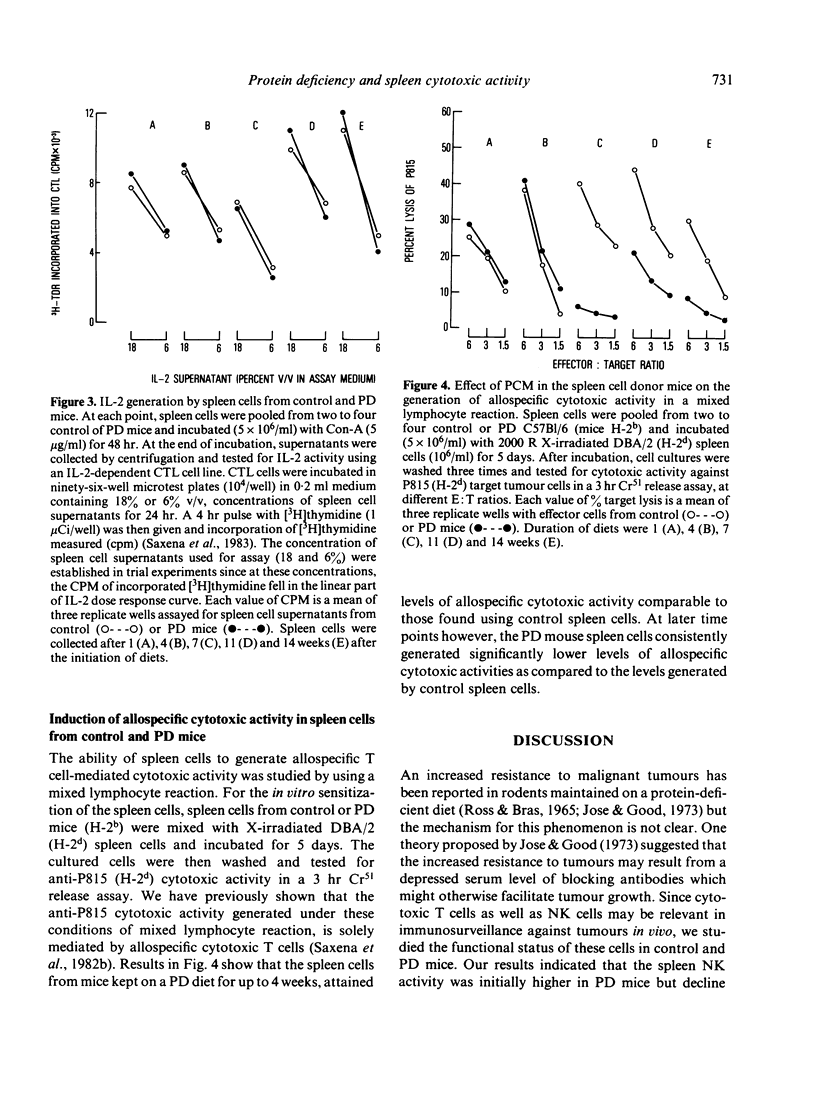
Six-week-old C57B1/6 female mice were fed a normal (24% protein) or an isocaloric but protein-deficient (4% protein) diet. At different time periods after the initiation of diets, basal natural killer (NK) activity, interleukin-2 (IL-2) and concanavalin-A (Con-A)-induced cytotoxic activity, Con-A-induced IL-2 production and levels of allospecific cytotoxic T cell activity generated in a mixed lymphocyte culture (MLC), were studied in spleen cells derived from control and protein deficient (PD) mice. Results indicated that (a) levels of spleen NK activity increased initially in PD mice, but after 7 weeks on PD diet declined to normal and subnormal levels, (b) IL-2 generation in response to Con-A as well as IL-2 activation of NK activity were comparable in spleen cells of control and PD mice at all time points tested, (c) Con-A-induced cytotoxic activity was significantly greater in spleen cells from PD mice, the difference being greater at higher doses of Con-A, and (d) generation of alloimmune cytotoxic T cells in a MLC reaction was normal in PD mouse spleen cells until 4 weeks after the beginning of PD diet, but declined markedly thereafter. Relevance of these observations to other related findings in protein calorie malnutrition are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLOUGH W. S., EISA E. A. The effects of a graded series of restricted diets on epidermal mitotic activity in the mouse. Br J Cancer. 1950 Sep;4(3):321–328. doi: 10.1038/bjc.1950.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet F. M. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Antibody formation in first and second generation offspring of nutritionally deprived rats. Science. 1975 Oct 17;190(4211):289–290. doi: 10.1126/science.1179211. [DOI] [PubMed] [Google Scholar]
- Demopoulos H. B. Effects of low phenylalanine-tyrosine diets on S91 mouse melanomas. J Natl Cancer Inst. 1966 Aug;37(2):185–190. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Smith K. A., Watson J. Biochemical characterization of lymphocyte regulatory molecules. II. Purification of a class of rat and human lymphokines. J Immunol. 1980 Apr;124(4):1954–1962. [PubMed] [Google Scholar]
- Hui Y. H., DeOme K. B., Briggs G. M. Inhibition of pituitary-induced nodular hyperplasia in mammary glands of C3H mice fed a phenylalanine-deficient diet. J Natl Cancer Inst. 1971 May;46(5):929–939. [PubMed] [Google Scholar]
- Jose D. G., Good R. A. Quantitative effects of nutritional protein and calorie deficiency upon immune responses to tumors in mice. Cancer Res. 1973 Apr;33(4):807–812. [PubMed] [Google Scholar]
- Keusch G. T. Host defense mechanisms in protein energy malnutrition. Adv Exp Med Biol. 1981;135:183–209. doi: 10.1007/978-1-4615-9200-6_10. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Petranyi G., Kärre K., Jondal M., Tracey D., Wigzell H. Killer cells: a functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J Exp Med. 1976 Apr 1;143(4):772–780. doi: 10.1084/jem.143.4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavé I., Layrisse M. Immune response in malnutrition. Differential effect of dietary protein restriction on the IgM and IgG response to alloantigens. Cell Immunol. 1976 Feb;21(2):337–343. doi: 10.1016/0008-8749(76)90061-7. [DOI] [PubMed] [Google Scholar]
- Malavé I., Németh A., Pocino M. Changes in lymphocyte populations in protein--calorie-deficient mice. Cell Immunol. 1980 Feb;49(2):235–249. doi: 10.1016/0008-8749(80)90026-x. [DOI] [PubMed] [Google Scholar]
- Malavé I., Pocino M., Baute L. Influence of dietary protein restriction on the delayed-type hypersensitivity response to sheep red blood cells in mice. Immunology. 1983 Feb;48(2):329–336. [PMC free article] [PubMed] [Google Scholar]
- McFarlane H., Hamid J. Cell-mediated immune response in malnutrition. Clin Exp Immunol. 1973 Jan;13(1):153–164. [PMC free article] [PubMed] [Google Scholar]
- Narayanan R. B., Nath I., Bhuyan U. N., Talwar G. P. Depression of T-cell function and normality of B-cell response in protein calorie malnutrition. Immunology. 1977 Mar;32(3):345–350. [PMC free article] [PubMed] [Google Scholar]
- Pocino M., Malavé I. Enhancement of the in vitro antibody response in dietary protein restriction. Failure in the regulation of antibody synthesis. Immunology. 1981 Jun;43(2):235–240. [PMC free article] [PubMed] [Google Scholar]
- Price P., Turner K. J. Regulatory T cells in the humoral response of protein deficient mice. Clin Exp Immunol. 1979 Jan;35(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- Ross M. H., Bras G. Tumor incidence patterns and nutrition in the rat. J Nutr. 1965 Nov;87(3):245–260. doi: 10.1093/jn/87.3.245. [DOI] [PubMed] [Google Scholar]
- Salimonu L. S., Ojo-Amaize E., Williams A. I., Johnson A. O., Cooke A. R., Adekunle F. A., Alm G. V., Wigzell H. Depressed natural killer cell activity in children with protein-calorie malnutrition. Clin Immunol Immunopathol. 1982 Jul;24(1):1–7. doi: 10.1016/0090-1229(82)90082-4. [DOI] [PubMed] [Google Scholar]
- Saxena Q. B., Saxena R. K., Adler W. H. Regulation of natural killer activity in vivo. III. Effect of hypophysectomy and growth hormone treatment on the natural killer activity of the mouse spleen cell population. Int Arch Allergy Appl Immunol. 1982;67(2):169–174. [PubMed] [Google Scholar]
- Saxena R. K., Adler W. H. Modulation of natural cytotoxicity by alloantibodies. I. Alloantisera enhancement of cytotoxicity of mouse spleen cells toward a human myeloid cell line. J Immunol. 1979 Aug;123(2):846–851. [PubMed] [Google Scholar]
- Saxena R. K., Adler W. H., Nordin A. A. Modulation of natural cytotoxicity by alloantibodies. IV. A comparative study of the activation of mouse spleen cell cytotoxicity by anti H-2 antisera, interferon, and mitogens. Cell Immunol. 1981 Sep 1;63(1):28–41. doi: 10.1016/0008-8749(81)90026-5. [DOI] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Defective T-cell response in beige mutant mice. Nature. 1982 Jan 21;295(5846):240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Interleukin-2-induced activation of natural killer activity in spleen cells from old and young mice. Immunology. 1984 Apr;51(4):719–726. [PMC free article] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Collins G. D., Adler W. H. Augmentation of spleen natural killer activity in mice treated with interleukin-2 preparation. Indian J Exp Biol. 1983 Feb;21(2):54–58. [PubMed] [Google Scholar]



