Abstract
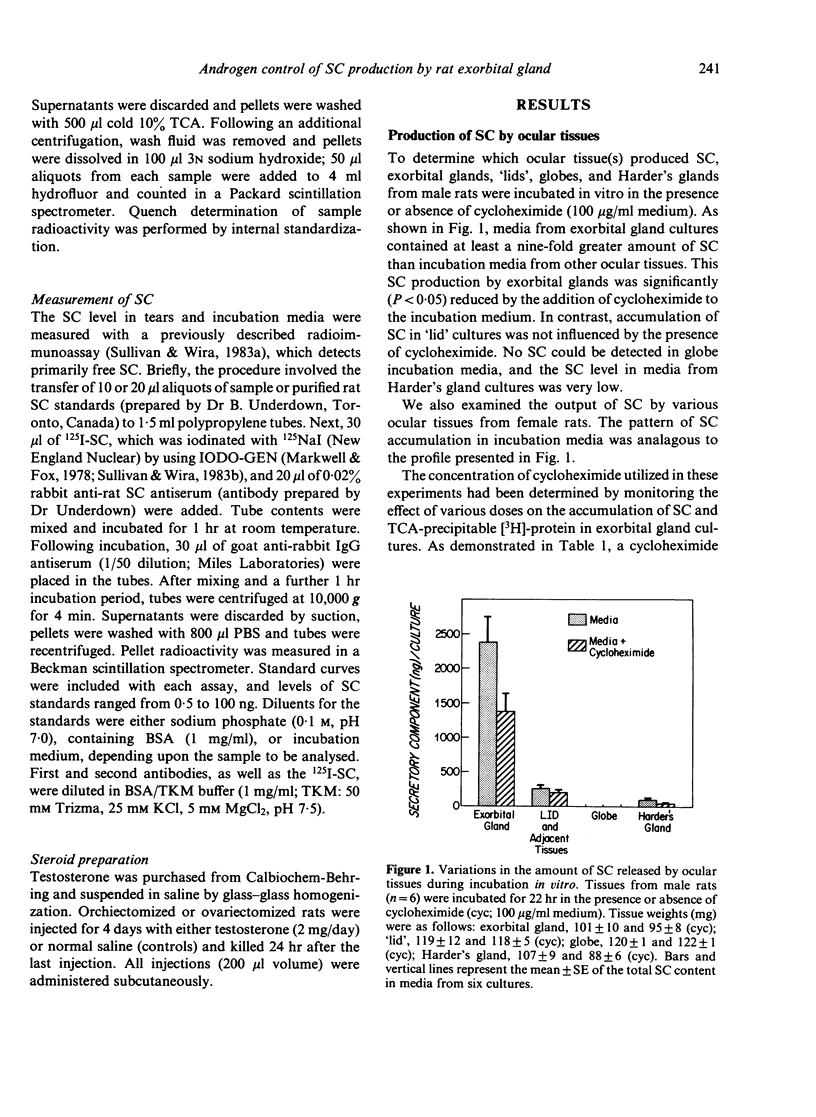
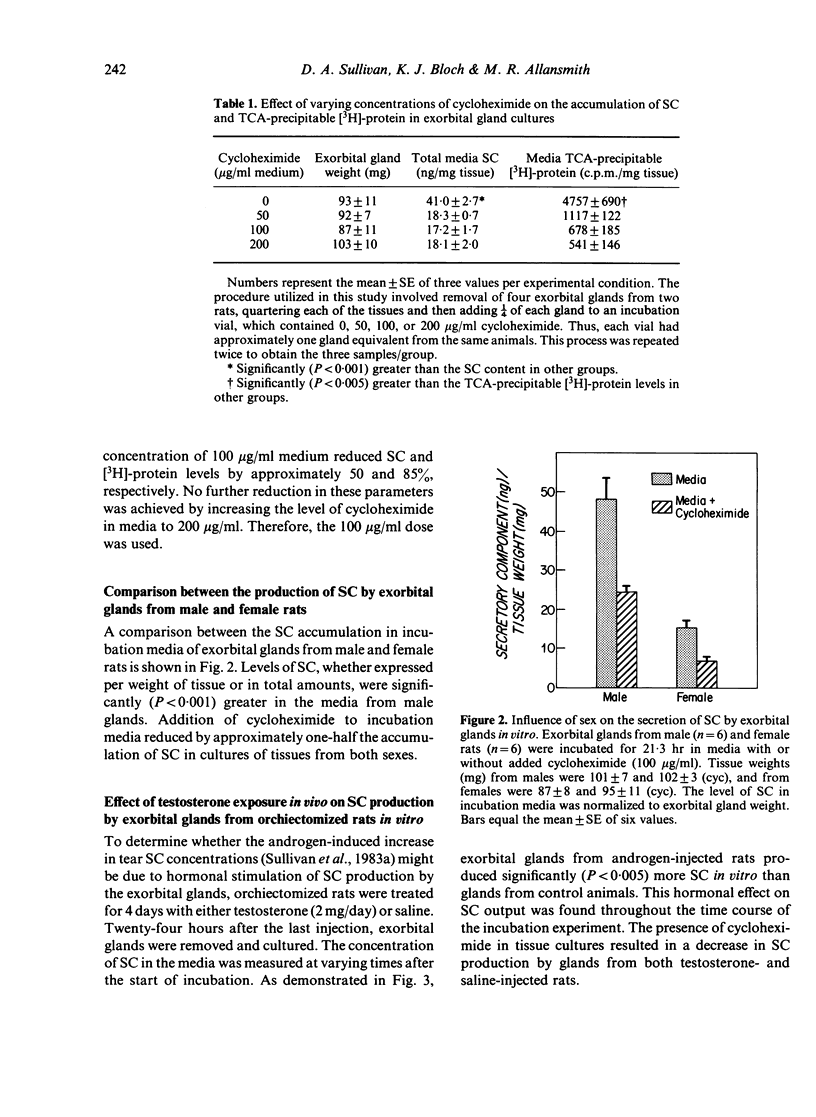
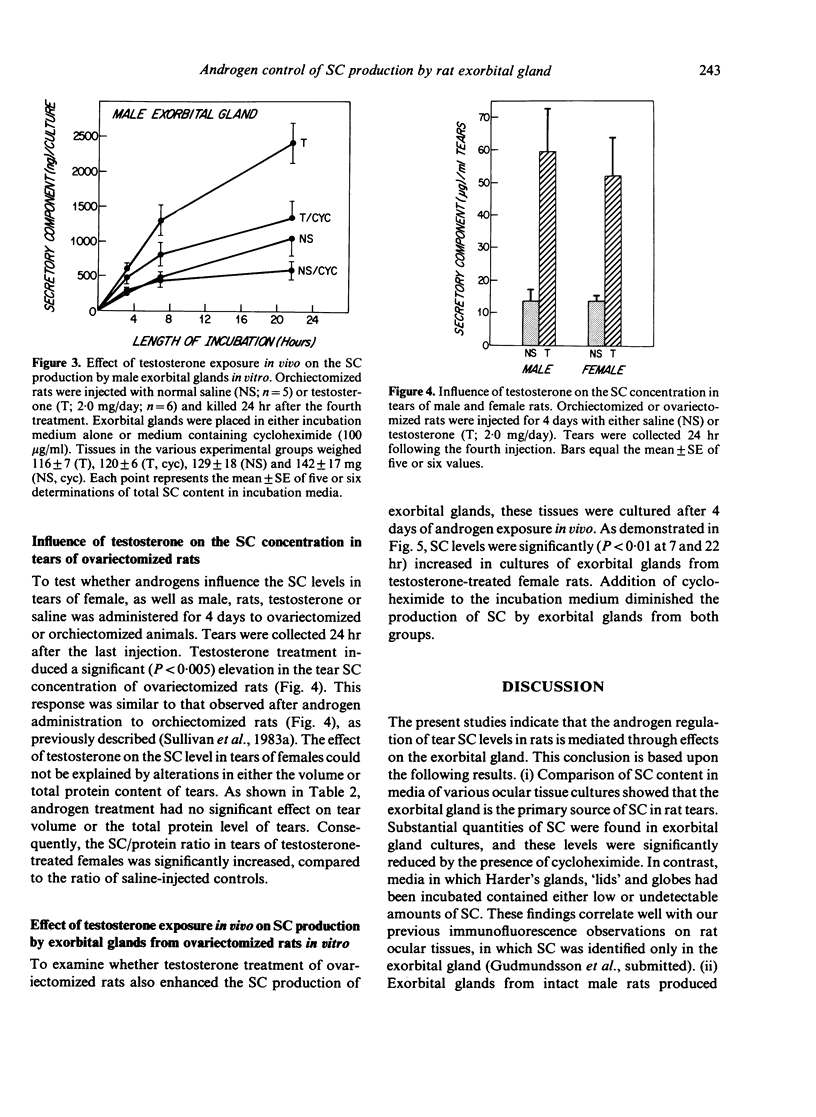
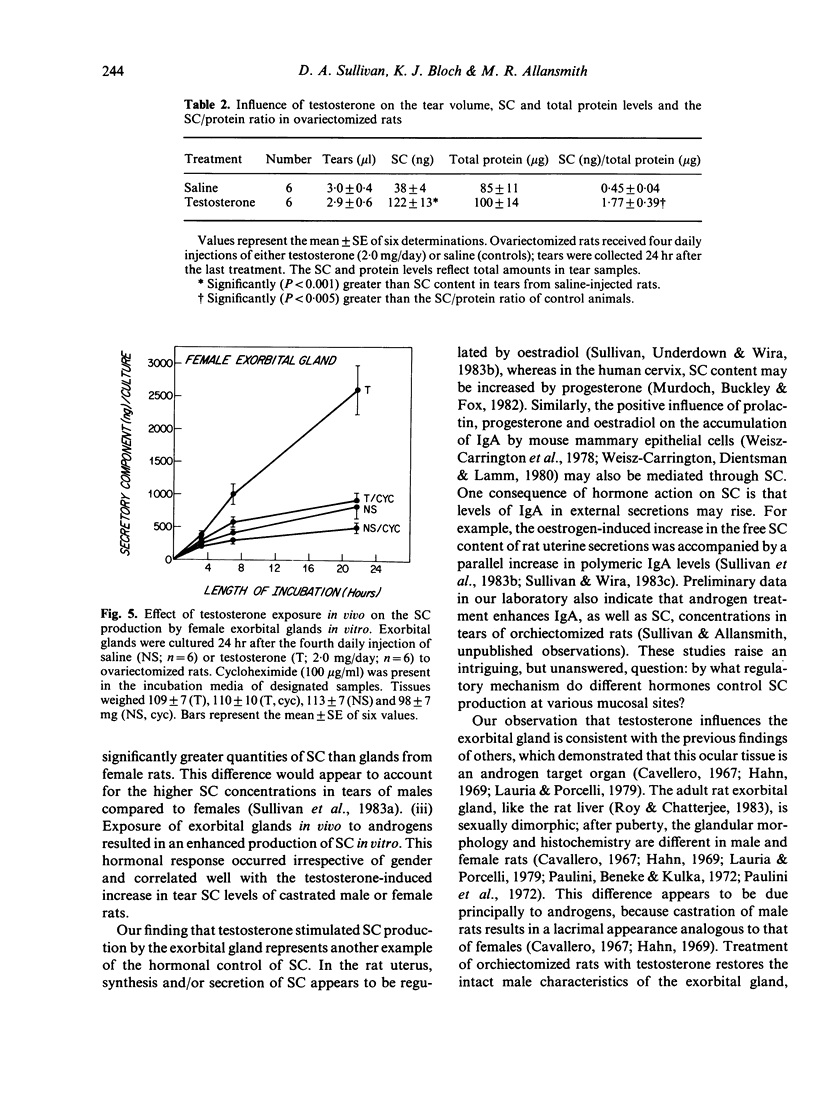
Androgens are known to regulate the level of secretory component (SC) in tears of male rats. The purpose of the present study was to explore the underlying mechanism of this hormone action by (i) identifying the ocular tissue(s) involved in SC production; and (ii) determining whether androgens increase SC production by this tissue. We also examined whether androgen administration influenced the concentration of SC in tears of female rats. Ocular tissues from adult Sprague-Dawley rats were cultured in the presence or absence of cycloheximide in the incubation medium. Secretory component in the culture media was measured by an RIA which detects primarily free SC. Analysis of media obtained after incubation of exorbital (lacrimal) glands, 'lid' tissues, globes, and Harderian glands revealed that only exorbital glands released substantial amounts of SC. This exorbital gland production of SC, which was significantly greater in tissues from male rats, as compared to those of female rats, was reduced by approximately 50% when cycloheximide was present in the culture medium. To determine whether SC production by exorbital glands was influenced by androgens, orchiectomized glands was influenced by androgens, orchiectomized rats were administered either saline or testosterone (2.0 mg/day for 4 days), and exorbital glands were cultured 24 hr after the last injection. Testosterone treatment in vivo induced a significant, cycloheximide-sensitive increase in SC production in vitro, compared to the glandular SC output of saline-injected controls. It is interesting that similar androgen treatment of ovariectomized females also resulted in elevated tear SC concentrations and enhanced output of SC by their exorbital glands in vitro. These findings indicate that the exorbital gland is primarily responsible for SC production in the rat eye and that androgens may modulate the synthesis of SC in this gland.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- BLOCH K. J., BUCHANAN W. W., WOHL M. J., BUNIM J. J. SJOEGREN'S SYNDROME. A CLINICAL, PATHOLOGICAL, AND SEROLOGICAL STUDY OF SIXTY-TWO CASES. Medicine (Baltimore) 1965 May;44:187–231. [PubMed] [Google Scholar]
- Brandtzaeg P. Transport models for secretory IgA and secretory IgM. Clin Exp Immunol. 1981 May;44(2):221–232. [PMC free article] [PubMed] [Google Scholar]
- Bromberg B. B. Autonomic control of lacrimal protein secretion. Invest Ophthalmol Vis Sci. 1981 Jan;20(1):110–116. [PubMed] [Google Scholar]
- CAVALLERO C., CHIAPPINO G., MILANI F., CASELLA E. Uptake of 35S labelled sulfate in the exorbital lacrymal glands of adult and newborn rats under different hormonal treatment. Experientia. 1960 Sep 15;16:429–429. doi: 10.1007/BF02178854. [DOI] [PubMed] [Google Scholar]
- Cavallero C. Relative effectiveness of varous steroids in an androgen assay using the exorbital lacrimal gland of the castrated rat. I. Delta-4-3-ketones and delta-5-3-beta-hydroxysteroids. Acta Endocrinol (Copenh) 1967 May;55(1):119–130. [PubMed] [Google Scholar]
- Crago S. S., Kulhavy R., Prince S. J., Mestecky J. Inhibition of the pokeweed mitogen-induced response of normal peripheral blood lymphocytes by humoral components of colostrum. Clin Exp Immunol. 1981 Aug;45(2):386–392. [PMC free article] [PubMed] [Google Scholar]
- Crago S. S., Kulhavy R., Prince S. J., Mestecky J. Secretory component of epithelial cells is a surface receptor for polymeric immunoglobulins. J Exp Med. 1978 Jun 1;147(6):1832–1837. doi: 10.1084/jem.147.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Delacroix D. L., Gharavi A. E., Vaerman J. P., Hughes G. R. Immunoglobulin A and polymeric IgA rheumatoid factors in systemic sicca syndrome: partial characterization. J Immunol. 1982 Aug;129(2):576–581. [PubMed] [Google Scholar]
- Elkon K. B., Gharavi A. E., Patel B. M., Hughes G. R., Frankel A. IgA and IgM rheumatoid factors in serum, saliva and other secretions: relationship to immunoglobulin ratios in systemic sicca syndrome and rheumatoid arthritis. Clin Exp Immunol. 1983 Apr;52(1):75–84. [PMC free article] [PubMed] [Google Scholar]
- Engstrom J. F., Arvanitakis C., Sagawa A., Abdou N. I. Secretory immunoglobulin deficiency in a family with inflammatory bowel disease. Gastroenterology. 1978 Apr;74(4):747–751. [PubMed] [Google Scholar]
- Hahn J. D. Effect of cyproterone acetate on sexual dimorphism of the exorbital lacrimal gland in rats. J Endocrinol. 1969 Nov;45(3):421–424. doi: 10.1677/joe.0.0450421. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Jahn R., Padel U., Porsch P. H., Söling H. D. Adrenocorticotropic hormone and alpha-melanocyte-stimulating hormone induce secretion and protein phosphorylation in the rat lacrimal gland by activation of a cAMP-dependent pathway. Eur J Biochem. 1982 Sep 1;126(3):623–629. doi: 10.1111/j.1432-1033.1982.tb06826.x. [DOI] [PubMed] [Google Scholar]
- Lauria A., Porcelli F. Leucine aminopeptidase (LAP) activity and sexual dimorphism in rat exorbital lacrimal gland. Basic Appl Histochem. 1979;23(2):171–177. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Michalski J. P., McCombs C. C., Roubinian J. R., Talal N. Effect of androgen therapy on survival and suppressor cell activity in aged NZB/NZW F1 hybrid mice. Clin Exp Immunol. 1983 Apr;52(1):229–233. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Nadler L. M., Distaso J. A., Steinberg A. D., Schlossman S. F. Comparison in T- and B-cell markers in patients with Sjögren's syndrome and systemic lupus erythematosus. Clin Immunol Immunopathol. 1982 Feb;22(2):270–278. doi: 10.1016/0090-1229(82)90043-5. [DOI] [PubMed] [Google Scholar]
- Murdoch A. J., Buckley C. H., Fox H. Hormonal control of the secretory immune system of the human uterine cervix. J Reprod Immunol. 1982 Feb;4(1):23–30. doi: 10.1016/0165-0378(82)90020-1. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Ogra S. S., Coppola P. R. Secretory component and sudden-infant-death syndrome. Lancet. 1975 Aug 30;2(7931):387–390. doi: 10.1016/s0140-6736(75)92898-6. [DOI] [PubMed] [Google Scholar]
- Peppard J. V., Orlans E., Andrew E., Payne A. W. Elimination into bile of circulating antigen by endogenous IgA antibody in rats. Immunology. 1982 Mar;45(3):467–472. [PMC free article] [PubMed] [Google Scholar]
- Roy A. K., Chatterjee B. Sexual dimorphism in the liver. Annu Rev Physiol. 1983;45:37–50. doi: 10.1146/annurev.ph.45.030183.000345. [DOI] [PubMed] [Google Scholar]
- Socken D. J., Simms E. S., Nagy B., Fisher M. M., Underdown B. J. Transport of IgA antibody-antigen complexes by the rat liver. Mol Immunol. 1981 Apr;18(4):345–348. doi: 10.1016/0161-5890(81)90059-6. [DOI] [PubMed] [Google Scholar]
- Stafford H. A., Knight K. L., Fanger M. W. Receptors for IgA on rabbit lymphocytes. II. Characterization of their binding parameters for IgA. J Immunol. 1982 May;128(5):2201–2205. [PubMed] [Google Scholar]
- Stimson W. H., Crilly P. J. Effects of steroids on the secretion of immunoregulatory factors by thymic epithelial cell cultures. Immunology. 1981 Oct;44(2):401–407. [PMC free article] [PubMed] [Google Scholar]
- Stokes C. R., Swarbrick E. T., Soothill J. F. Immune elimination and enhanced antibody responses: functions of circulating IgA. Immunology. 1980 Jul;40(3):455–458. [PMC free article] [PubMed] [Google Scholar]
- Strober W., Krakauer R., Klaeveman H. L., Reynolds H. Y., Nelson D. L. Secretory component deficiency. A disorder of the IgA immune system. N Engl J Med. 1976 Feb 12;294(7):351–356. doi: 10.1056/NEJM197602122940701. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Underdown B. J., Wira C. R. Steroid hormone regulation of free secretory component in the rat uterus. Immunology. 1983 Jun;49(2):379–386. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Hormonal regulation of immunoglobulins in the rat uterus: uterine response to a single estradiol treatment. Endocrinology. 1983 Jan;112(1):260–268. doi: 10.1210/endo-112-1-260. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Variations in free secretory component levels in mucosal secretions of the rat. J Immunol. 1983 Mar;130(3):1330–1335. [PubMed] [Google Scholar]
- Talal N., Dauphinee M. J., Wofsy D. Interleukin-2 deficiency, genes, and systemic lupus erythematosus. Arthritis Rheum. 1982 Jul;25(7):838–842. doi: 10.1002/art.1780250725. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., Phillips-Quagliata J. M., Lamm M. E. Hormonal induction of the secretory immune system in the mammary gland. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2928–2932. doi: 10.1073/pnas.75.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenbergen J. L., May C., Wanson J. C., Vaerman J. P. Synthesis of secretory component by rat hepatocytes in culture. Scand J Immunol. 1980;11(1):93–97. doi: 10.1111/j.1365-3083.1980.tb00213.x. [DOI] [PubMed] [Google Scholar]


