Abstract
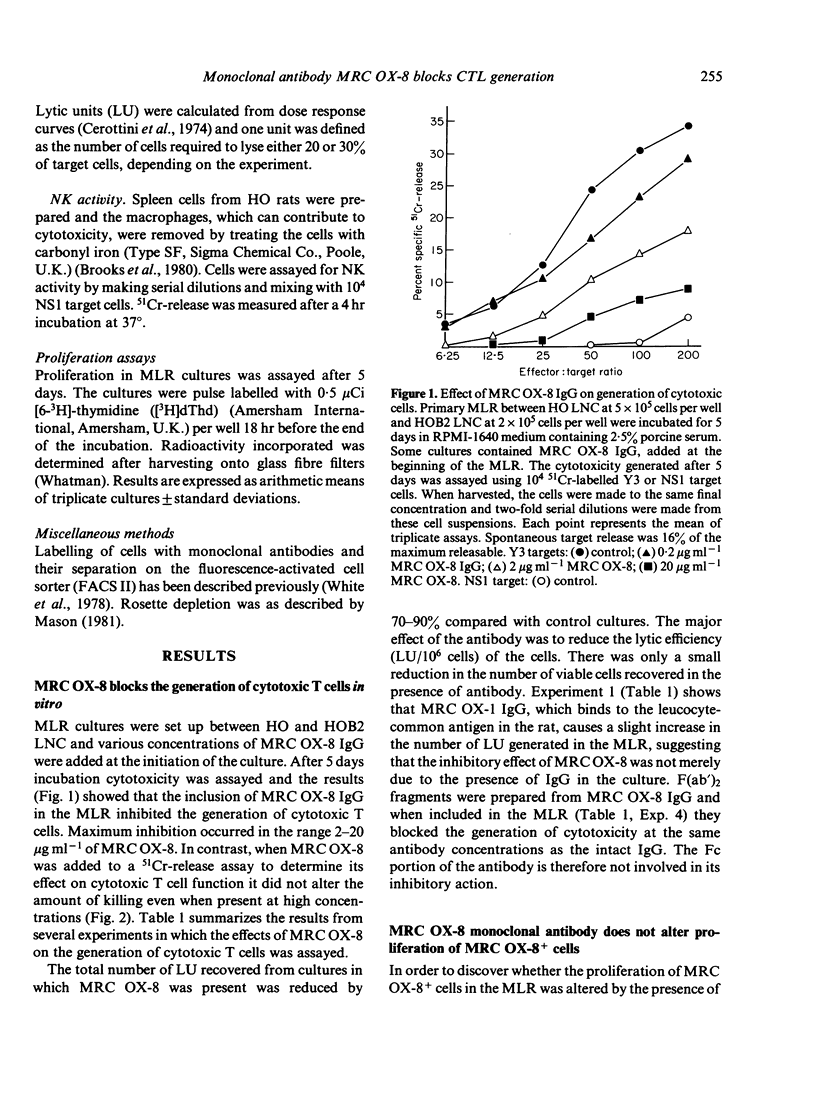
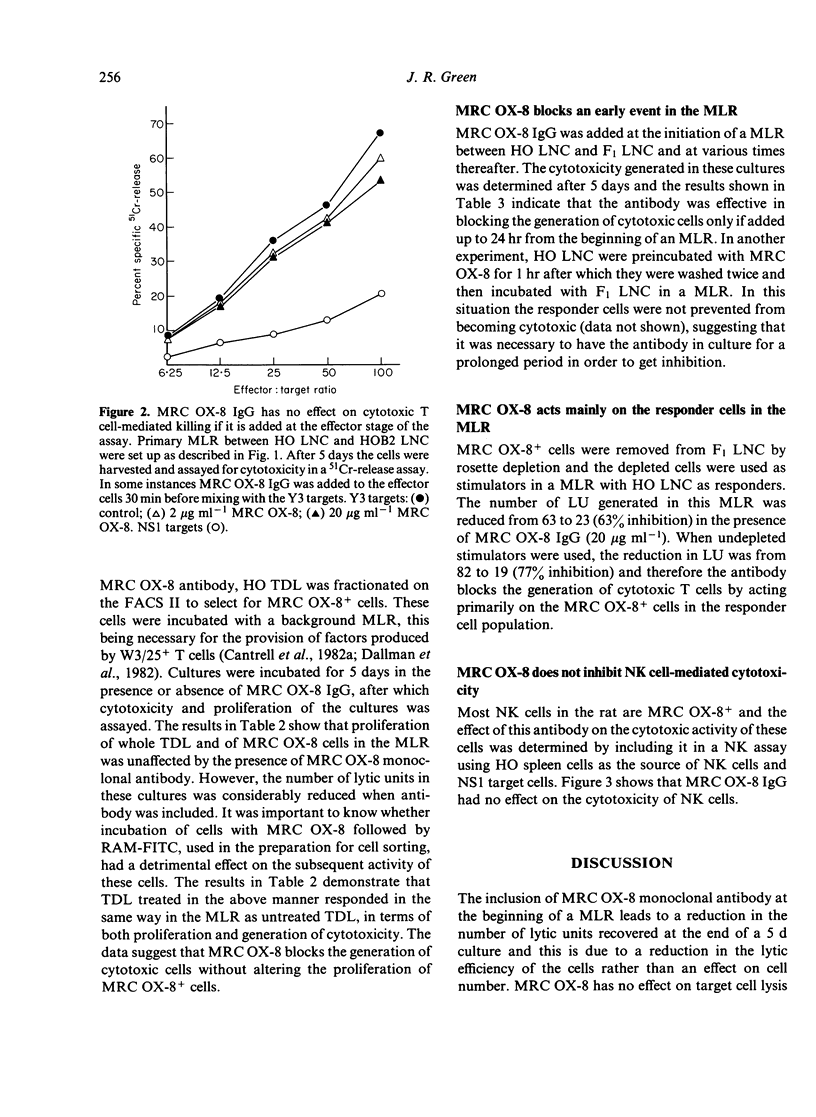
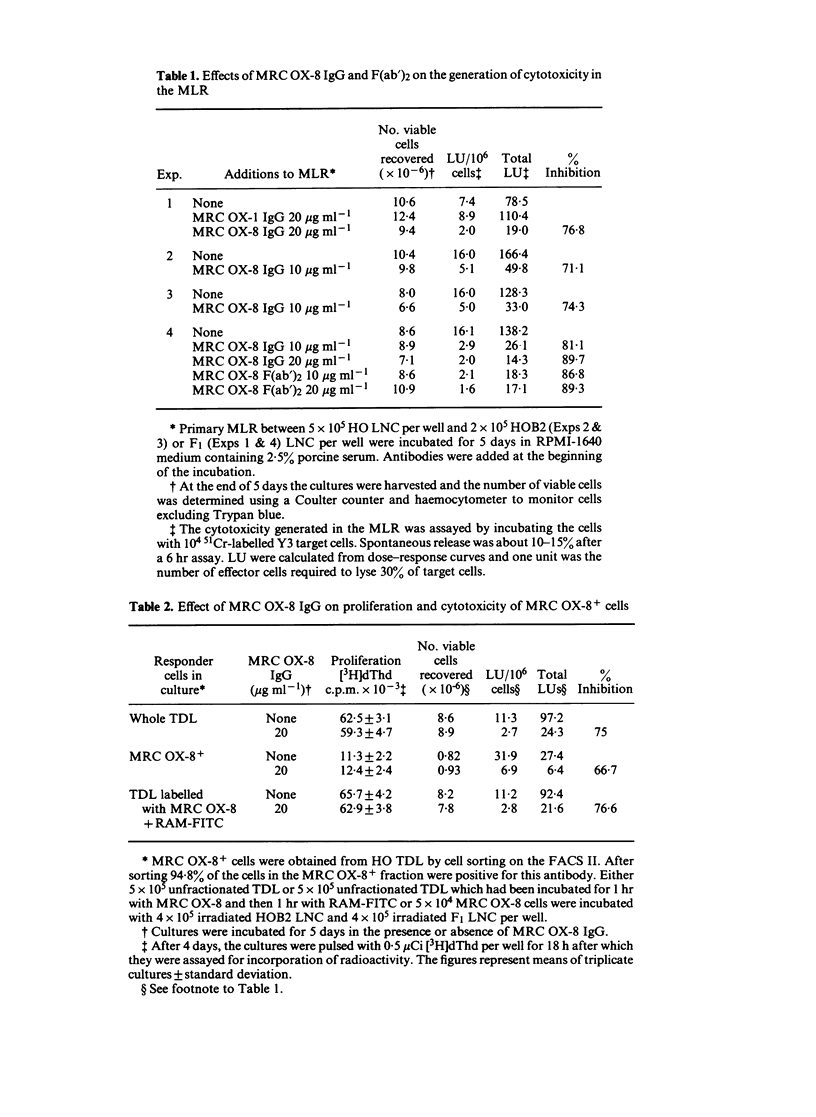
MRC OX-8 is a mouse anti-rat monoclonal antibody which binds to thymocytes, cytotoxic T cells, suppressor cells and the majority of natural killer (NK) cells. Addition of this antibody at the beginning of a mixed lymphocyte reaction (MLR) inhibits the generation of cytotoxic T cells, assayed after 5 days. However, MRC OX-8 antibody has no effect on proliferation in the MLR, in particular of the MRC OX-8+ cells. The generation of cytotoxicity in the MLR is blocked by MRC OX-8 IgG and F(ab')2 and requires interaction of responder cells with the antibody during the first 24 hr of the MLR, indicating that it is the early stages which are most affected. MRC OX-8 has no effect on cytotoxic T cell function when added at the effector stage of the 51Cr-release assay and has no effect on NK cell-mediated killing.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Biddison W. E., Rao P. E., Talle M. A., Goldstein G., Shaw S. Possible involvement of the OKT4 molecule in T cell recognition of class II HLA antigens. Evidence from studies of cytotoxic T lymphocytes specific for SB antigens. J Exp Med. 1982 Oct 1;156(4):1065–1076. doi: 10.1084/jem.156.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Flannery G. R., Webb P. J., Baldwin R. W. Quantitative studies of natural immunity to solid tumours in rats. The nature of the killer cell depends on the type of assay. Immunology. 1980 Nov;41(3):673–680. [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Robins R. A., Baldwin R. W. Rat lymphocyte subsets: cellular requirements for the generation of T-cell growth factor. Cell Immunol. 1982 Jul 1;70(2):367–372. doi: 10.1016/0008-8749(82)90338-0. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- Davignon D., Martz E., Reynolds T., Kürzinger K., Springer T. A. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4535–4539. doi: 10.1073/pnas.78.7.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. C., Rosenberg J. S., Feldman J. D. Membrane phenotype of the rat cytotoxic T lymphocyte. J Immunol. 1982 Sep;129(3):1012–1016. [PubMed] [Google Scholar]
- Haskins K., Kubo R., White J., Pigeon M., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983 Apr 1;157(4):1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Hollander N., Pillemer E., Weissman I. L. Effects of Lyt antibodies on T-cell functions: augmentation by anti-Lyt-1 as opposed to inhibition by anti-Lyt-2. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1148–1151. doi: 10.1073/pnas.78.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancki D. W., Lorber M. I., Loken M. R., Fitch F. W. A clone-specific monoclonal antibody that inhibits cytolysis of a cytolytic T cell clone. J Exp Med. 1983 Mar 1;157(3):921–935. doi: 10.1084/jem.157.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lyscom N., Brueton M. J. Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology. 1982 Apr;45(4):775–783. [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Bron C., Kelso A., Cerottini J. C. Clonal heterogeneity in the functional requirement for Lyt-2/3 molecules on cytolytic T lymphocytes (CTL): possible implications for the affinity of CTL antigen receptors. Immunol Rev. 1982;68:89–115. doi: 10.1111/j.1600-065x.1982.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Mason D. W. Subsets of T cells in the rat mediating lethal graft versus-host disease. Transplantation. 1981 Sep;32(3):222–226. doi: 10.1097/00007890-198109000-00008. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Hodgdon J. C., Hercend T., Schlossman S. F., Reinherz E. L. Surface structures involved in target recognition by human cytotoxic T lymphocytes. Science. 1982 Oct 29;218(4571):471–473. doi: 10.1126/science.6981845. [DOI] [PubMed] [Google Scholar]
- Männel D. N., Falk W., Dröge W. Induction of cytotoxic T cell function requires sequential action of three different lymphokines. J Immunol. 1983 Jun;130(6):2508–2510. [PubMed] [Google Scholar]
- Osawa H., Diamantstein T. The characteristics of a monoclonal antibody that binds specifically to rat T lymphoblasts and inhibits IL 2 receptor functions. J Immunol. 1983 Jan;130(1):51–55. [PubMed] [Google Scholar]
- Pearson G. R., Hodes R. J., Friberg S. Cytotoxic potential of different lymphoid cell populations against chromium-51 labelled tumour cells. Clin Exp Immunol. 1969 Sep;5(3):273–284. [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H., Bevan M. J. A differentiation factor required for the expression of cytotoxic T-cell function. Nature. 1982 Apr 22;296(5859):754–757. doi: 10.1038/296754a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Green J. R. Molecular nature of the W3/25 and MRC OX-8 marker antigens for rat T lymphocytes: comparisons with mouse and human antigens. Eur J Immunol. 1983 Oct;13(10):855–858. doi: 10.1002/eji.1830131014. [DOI] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Rouse B. T., Röllinghoff M., Scheurich P., Pfizenmaier K. Dissection of the proliferative and differentiative signals controlling murine cytotoxic T lymphocyte responses. J Exp Med. 1982 Jun 1;155(6):1876–1881. doi: 10.1084/jem.155.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M., Mason D. W., Williams A. F. Inhibition of mixed lymphocyte response by monoclonal antibody specific for a rat T lymphocyte subset. Nature. 1979 Dec 20;282(5741):841–843. doi: 10.1038/282841a0. [DOI] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]


