Abstract
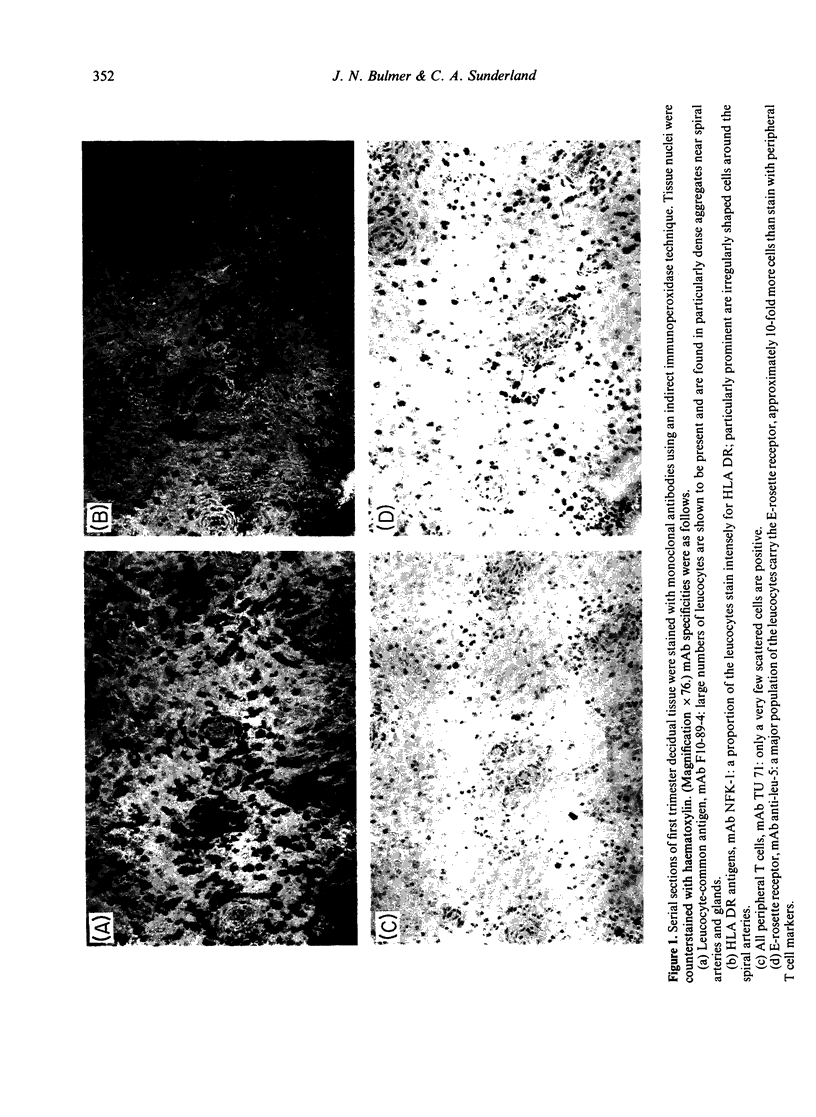
The distribution of leukocytes in first trimester human decidual tissue has been studied by using a panel of monoclonal antibodies in an indirect immunoperoxidase technique on acetone-fixed cryostat sections. The results indicate that bone marrow-derived cells are abundant in the placental bed and a proportion of these are HLA-DR positive. However, a major leukocyte population in the decidua of early pregnancy is of cells which carry the E-rosette receptor but which do not express peripheral pan-T cell antigens nor HLA-DR. The distribution of these cells suggests that they are endometrial granulocytes. A similar large number of cells express OKT 10, a marker of immature or activated cells. The presence of this unusual T lineage cell raises the possibility that a form of lymphocyte processing is occurring in the decidua in early pregnancy, perhaps in response to foetal antigens presented on trophoblast.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Bulmer J. N., Billington W. D., Johnson P. M. Immunohistologic identification of trophoblast populations in early human pregnancy with the use of monoclonal antibodies. Am J Obstet Gynecol. 1984 Jan 1;148(1):19–26. doi: 10.1016/s0002-9378(84)80026-5. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Sunderland C. A. Bone-marrow origin of endometrial granulocytes in the early human placental bed. J Reprod Immunol. 1983 Nov;5(6):383–387. doi: 10.1016/0165-0378(83)90247-4. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification with a monoclonal antibody of a predominantly B lymphocyte-specific determinant of the human leukocyte common antigen. Evidence for structural and possible functional diversity of the human leukocyte common molecule. J Exp Med. 1981 Apr 1;153(4):753–765. doi: 10.1084/jem.153.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuggle S. V., Errasti P., Daar A. S., Fabre J. W., Ting A., Morris P. J. Localization of major histocompatibility complex (HLA-ABC and DR) antigens in 46 kidneys. Differences in HLA-DR staining of tubules among kidneys. Transplantation. 1983 Apr;35(4):385–390. doi: 10.1097/00007890-198304000-00024. [DOI] [PubMed] [Google Scholar]
- Golander A., Zakuth V., Shechter Y., Spirer Z. Suppression of lymphocyte reactivity in vitro by a soluble factor secreted by explants of human decidua. Eur J Immunol. 1981 Oct;11(10):849–851. doi: 10.1002/eji.1830111020. [DOI] [PubMed] [Google Scholar]
- Howard F. D., Ledbetter J. A., Wong J., Bieber C. P., Stinson E. B., Herzenberg L. A. A human T lymphocyte differentiation marker defined by monoclonal antibodies that block E-rosette formation. J Immunol. 1981 Jun;126(6):2117–2122. [PubMed] [Google Scholar]
- Janossy G., Tidman N., Papageorgiou E. S., Kung P. C., Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981 Apr;126(4):1608–1613. [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A. H., Swettenham K. V., Vernon-Roberts B., Revell P. A. Studies on the function of the Kurloff cell. Int Arch Allergy Appl Immunol. 1971;40(1):137–152. doi: 10.1159/000230401. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Moretta A., Mingari M. C., Corte G., Moretta L. Receptors for immunoglobulins and activation markers on human T lymphocytes. Clin Haematol. 1982 Oct;11(3):697–709. [PubMed] [Google Scholar]
- Peel S., Bulmer D. The fine structure of the rat metrial gland in relation to the origin of the granulated cells. J Anat. 1977 Jul;123(Pt 3):687–696. [PMC free article] [PubMed] [Google Scholar]
- Peel S., Stewart I. J., Bulmer D. Experimental evidence for the bone marrow origin of granulated metrial gland cells of the mouse uterus. Cell Tissue Res. 1983;233(3):647–656. doi: 10.1007/BF00212232. [DOI] [PubMed] [Google Scholar]
- Slapsys R. M., Clark D. A. Active suppression of host-vs-graft reaction in pregnant mice. IV. Local suppressor cells in decidua and uterine blood. J Reprod Immunol. 1982 Dec;4(6):355–364. doi: 10.1016/0165-0378(82)90010-9. [DOI] [PubMed] [Google Scholar]
- Slapsys R., Clark D. A. Active suppression of host-versus-graft reaction in pregnant mice. V. Kinetics, specificity, and in vivo activity of non-T suppressor cells localized to the genital tract of mice during first pregnancy. Am J Reprod Immunol. 1983 Mar;3(2):65–71. doi: 10.1111/j.1600-0897.1983.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- Stewart I., Peel S. The structure and differentiation of granulated metrial gland cells of the pregnant mouse uterus. Cell Tissue Res. 1977 Nov 23;184(4):517–527. doi: 10.1007/BF00220975. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., Naiem M., Mason D. Y., Redman C. W., Stirrat G. M. The expression of major histocompatibility antigens by human chorionic villi. J Reprod Immunol. 1981 Dec;3(6):323–331. doi: 10.1016/0165-0378(81)90048-6. [DOI] [PubMed] [Google Scholar]
- Sutton L., Mason D. Y., Redman C. W. HLA-DR positive cells in the human placenta. Immunology. 1983 May;49(1):103–112. [PMC free article] [PubMed] [Google Scholar]
- Tekelioğlu-Uysal M., Edwards R. G., Kişnişi H. A. Ultrastructural relationships between decidua, trophoblast and lymphocytes at the beginning of human pregnancy. J Reprod Fertil. 1975 Mar;42(3):431–438. doi: 10.1530/jrf.0.0420431. [DOI] [PubMed] [Google Scholar]
- Terhorst C., van Agthoven A., LeClair K., Snow P., Reinherz E., Schlossman S. Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell. 1981 Mar;23(3):771–780. doi: 10.1016/0092-8674(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]






