Abstract
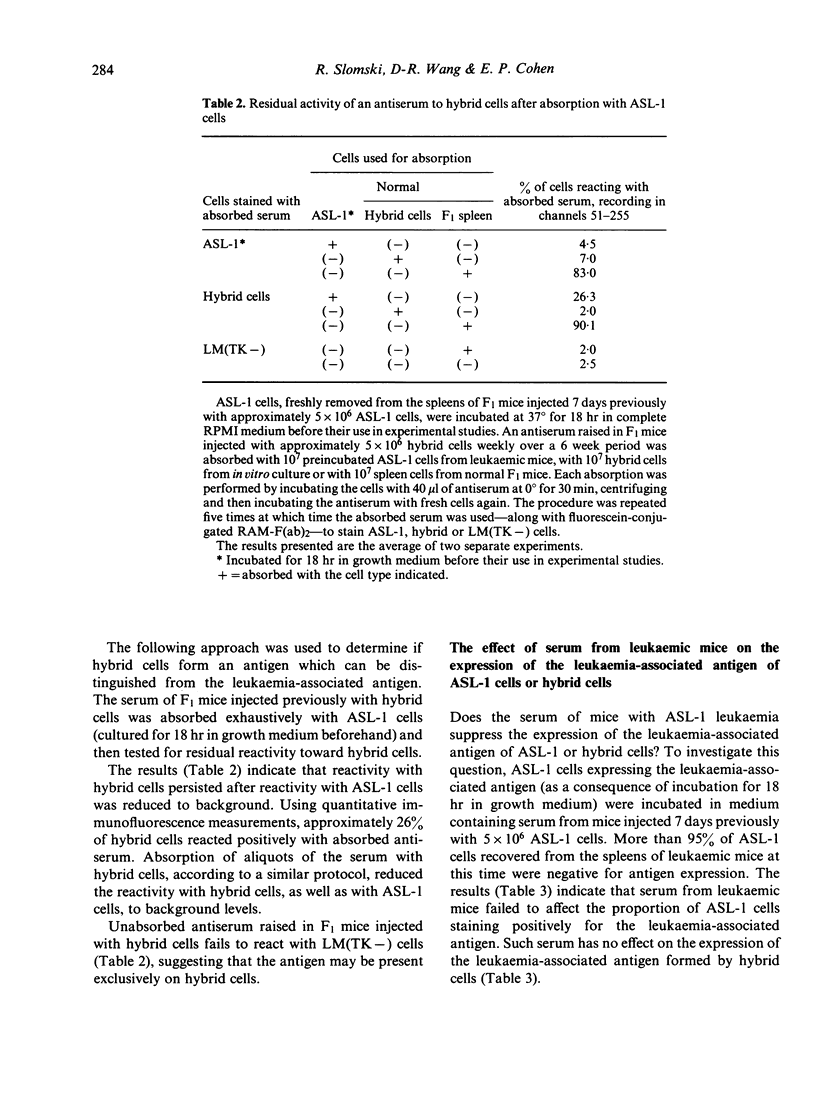
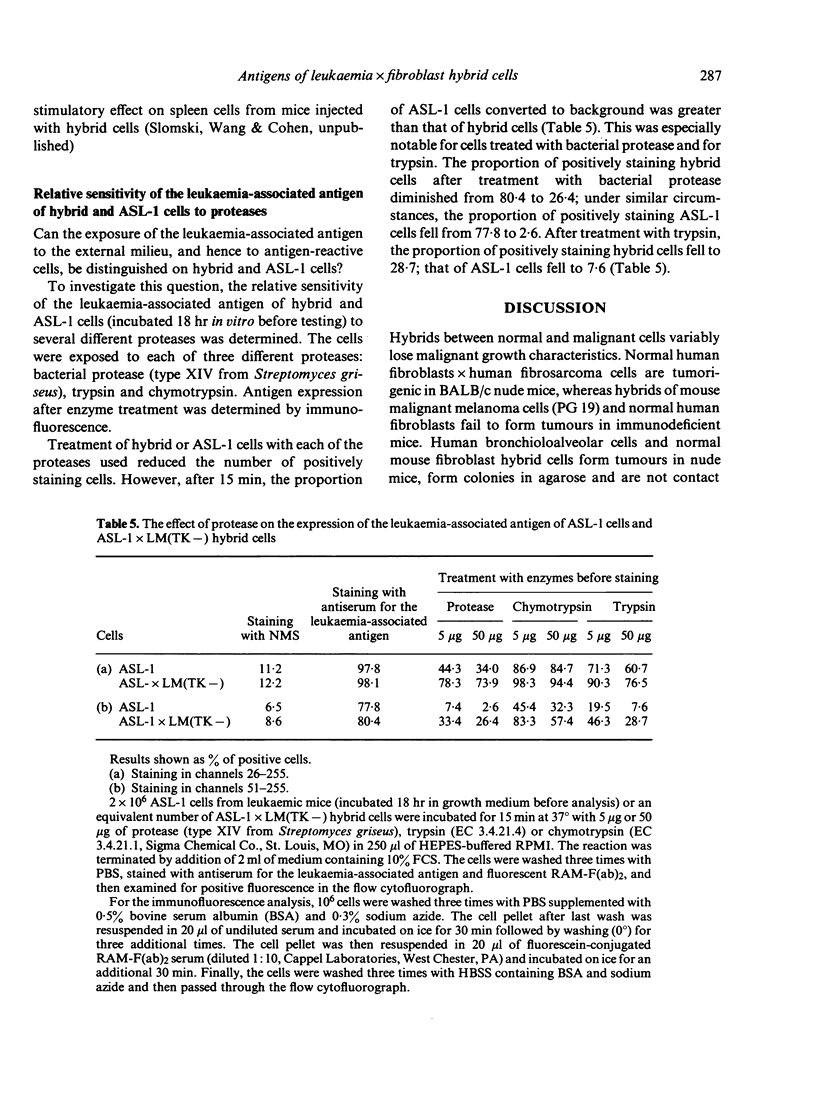
Somatic hybrids of ASL-1 leukaemia cells and LM(TK-) fibroblast cells, established mouse cell lines, are rejected by immunocompetent histocompatible mice; however, they grow progressively in nude mice and proliferate indefinitely in vitro. Mice rejecting such hybrid cells exhibit immunity toward ASL-1 leukaemia cells, used as one of the parents in forming the hybrid. In histocompatible recipients, ASL-1 cells are highly malignant, but LM(TK-) cells are rejected. Several distinguishing characteristics in the properties of the surface antigens of the three cell types are described. ASL-1 cells and hybrid cells but not LM(TK-) cells form an antigenically cross-reactive leukaemia-associated antigen; however, it is not detected on the surface membranes of ASL-1 cells taken directly from leukaemic mice. The antigen becomes apparent after short-term culture of the cells. Serum from leukaemic animals, unlike specific antibodies, has no effect upon the expression of the leukaemia-associated antigen of either hybrid or ASL-1 cells. Hybrid cells form a 'second' antigen, foreign to F1 mice, which can be distinguished from the leukaemia-associated antigen of ASL-1 cells. It is not detected on either parental cell. The leukaemia-associated antigen of ASL-1 cells is more readily digested by each of three proteases used than the analogous antigen of hybrid cells. The heightened immunogenic properties of hybrid cells is reflected by the observation that spleen cells from mice injected with hybrid cells undergo extensive proliferation in short-term in vitro culture, with or without an added specific stimulus.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barski G., Belehradek J., Jr Inheritance of malignancy in somatic cell hybrids. Somatic Cell Genet. 1979 Nov;5(6):897–908. doi: 10.1007/BF01542649. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Edgell C. J., Gazdar A. F., Minna J. D. Suppression of malignancy in human lung cancer (A549/8) times mouse fibroblast (3T3-4E) somatic cell hybrids. J Natl Cancer Inst. 1979 Feb;62(2):411–415. [PubMed] [Google Scholar]
- Colnaghi M. I. Histocompatibility antigens acting as helper determinants for tumor-associated antigens of murine lymphosarcoma. Eur J Immunol. 1975 Apr;5(4):241–245. doi: 10.1002/eji.1830050404. [DOI] [PubMed] [Google Scholar]
- Colten H. R., Parkman R. Biosynthesis of C4 (fourth component of complement) by hybrids of C4-deficient guinea pig cells and HeLa cells. Science. 1972 Jun 2;176(4038):1029–1031. doi: 10.1126/science.176.4038.1029. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Barrick J., Linnenbach A., Koprowski H. Expression of malignancy in hybrids between normal and malignant cells. J Cell Physiol. 1979 Jun;99(3):279–285. doi: 10.1002/jcp.1040990302. [DOI] [PubMed] [Google Scholar]
- Jami J., Ritz E. Nonmalignancy of hybrids derived from two mouse malignant cells. I. Hybrids between L1210 leukemia cells and malignant L cells. J Natl Cancer Inst. 1973 Nov;51(5):1647–1653. doi: 10.1093/jnci/51.5.1647. [DOI] [PubMed] [Google Scholar]
- Jami J., Ritz E. Tumor-associated transplantation antigens in immune rejection of mouse malignant cell hybrids. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2130–2134. doi: 10.1073/pnas.72.6.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson J., Harris H. The analysis of malignancy by cell fusion. VIII. Evidence for the intervention of an extra-chromosomal element. J Cell Sci. 1977 Apr;24:255–263. doi: 10.1242/jcs.24.1.255. [DOI] [PubMed] [Google Scholar]
- Jonasson J., Povey S., Harris H. The analysis of malignancy by cell fusion. VII. Cytogenetic analysis of hybrids between malignant and diploid cells and of tumours derived from them. J Cell Sci. 1977 Apr;24:217–254. doi: 10.1242/jcs.24.1.217. [DOI] [PubMed] [Google Scholar]
- Kim B. S., Liang W., Cohen E. P. Tumor-specific immunity induced by somatic hybrids. I. Lack of relationship between immunogenicity and tumorigenicity of selected hybrids. J Immunol. 1979 Aug;123(2):733–738. [PubMed] [Google Scholar]
- Kim B. S. Tumor-specific immunity induced by somatic hybrids. II. Elicitation of enhanced immunity against the parent plasmacytoma. J Immunol. 1979 Aug;123(2):739–744. [PubMed] [Google Scholar]
- Liang W., Cohen E. P. Activation of specific cellular immunity toward murine leukemia in mice rejecting syngeneic somatic hybrid cells. J Immunol. 1977 Sep;119(3):1054–1060. [PubMed] [Google Scholar]
- Liang W., Cohen E. P. Resistance to murine leukemia in mice receiving simultaneous injections of syngeneic hybrid and parental neoplastic cells. J Immunol. 1977 Mar;118(3):903–908. [PubMed] [Google Scholar]
- Liang W., Cohen E. P. Resistance to murine leukemia in mice rejecting syngeneic somatic hybrid cells. J Immunol. 1976 Mar;116(3):623–626. [PubMed] [Google Scholar]
- Louis C. J., Rose G. B. Suppression of malignancy in mouse-man hybrid cells. Pathology. 1978 Oct;10(4):343–350. doi: 10.3109/00313027809063523. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Shkolnik T., Sachs L. Suppression of the in vivo malignancy and in vitro cell multiplication of myeloid leukemic cells by hybridization with normal macrophages. Exp Cell Res. 1978 Apr;113(1):197–204. doi: 10.1016/0014-4827(78)90100-3. [DOI] [PubMed] [Google Scholar]
- Straus D. S., Jonasson J., Harris H. Growth in vitro of tumour cell x fibroblast hybrids in which malignancy is suppressed. J Cell Sci. 1977 Jun;25:73–86. doi: 10.1242/jcs.25.1.73. [DOI] [PubMed] [Google Scholar]
- Toffaletti D. L., Darrow T. L., Scott D. W. Augmentation of syngeneic tumor-specific immunity by semiallogeneic cell hybrids. J Immunol. 1983 Jun;130(6):2982–2986. [PubMed] [Google Scholar]
- Weiss M. C., Chaplain M. Expression of differentiated functions in hepatoma cell hybrids: reappearance of tyrosine aminotransferase inducibility after the loss of chromosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3026–3030. doi: 10.1073/pnas.68.12.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A., Cohen E. P. Studies on the effect of specific antisera on the metabolism of cellular antigens. I. Isolation of thymus leukemia antigens. J Immunol. 1974 Apr;112(4):1285–1295. [PubMed] [Google Scholar]
- Yu A., Cohen E. P. Studies on the effect of specific antisera on the metabolism of cellular antigens. II. The synthesis and degradation of TL antigens of mouse cells in the presence of TL antiserum. J Immunol. 1974 Apr;112(4):1296–1307. [PubMed] [Google Scholar]


