Abstract
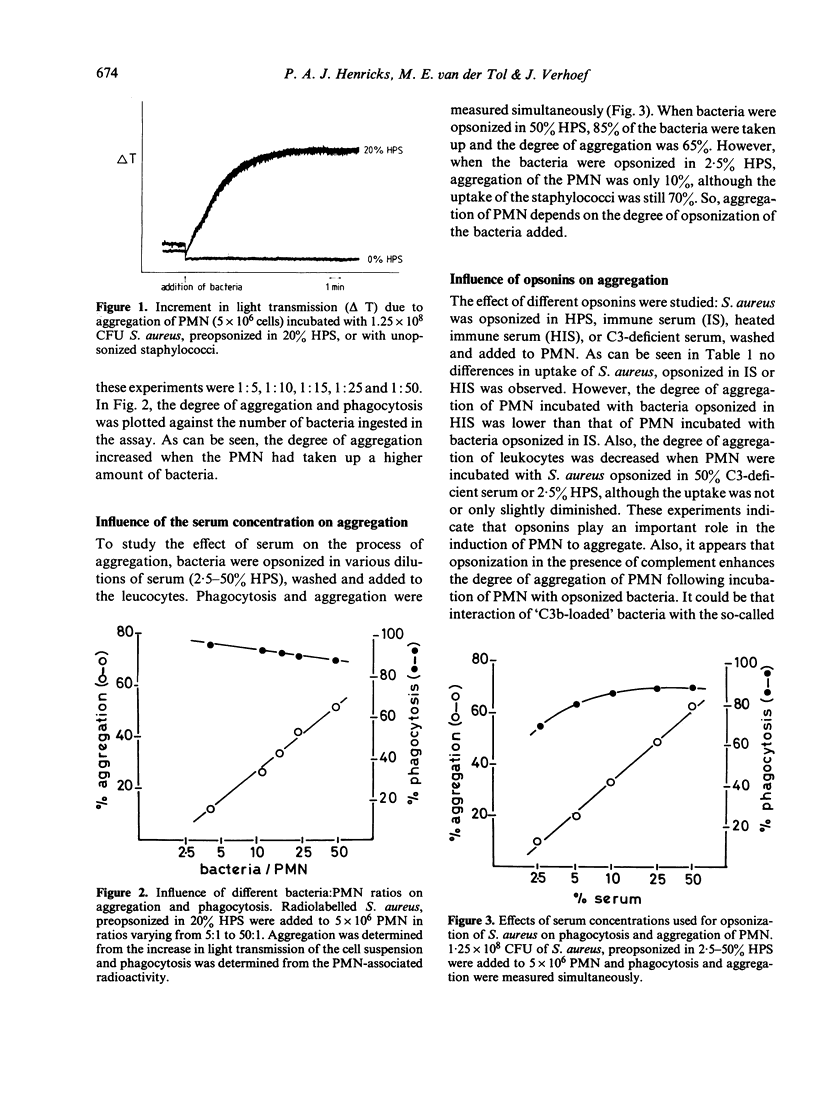
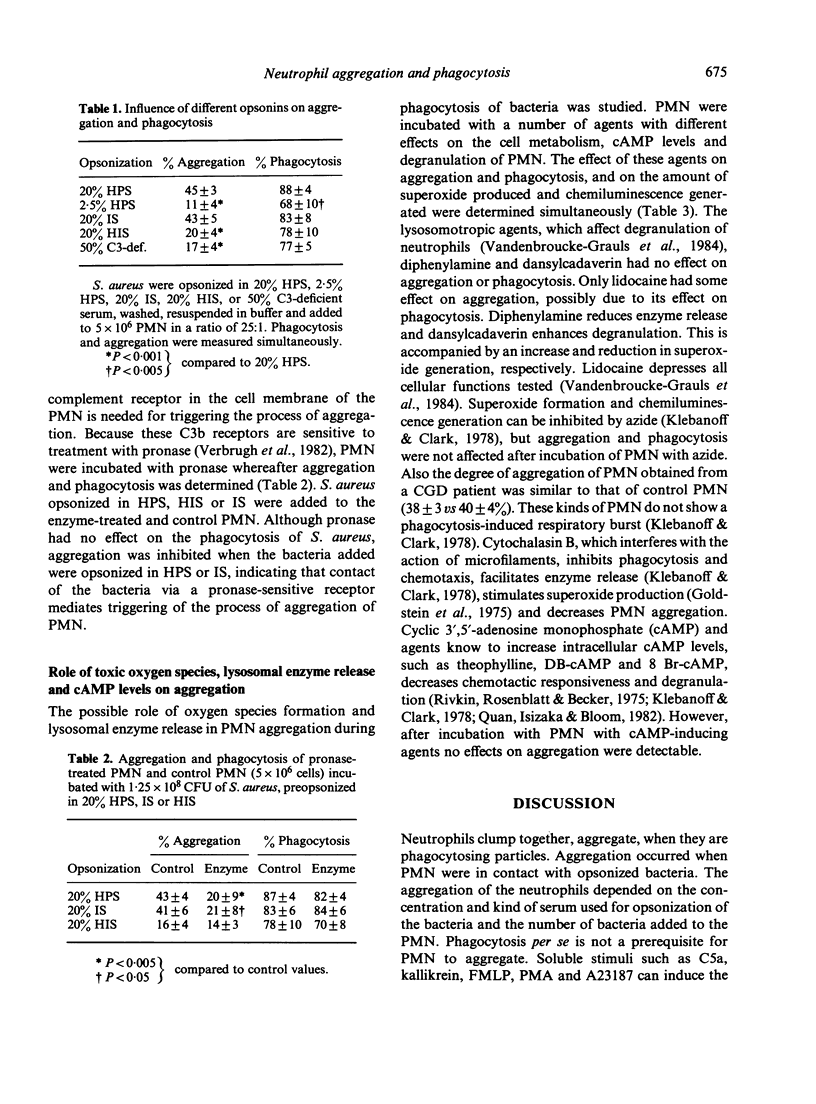
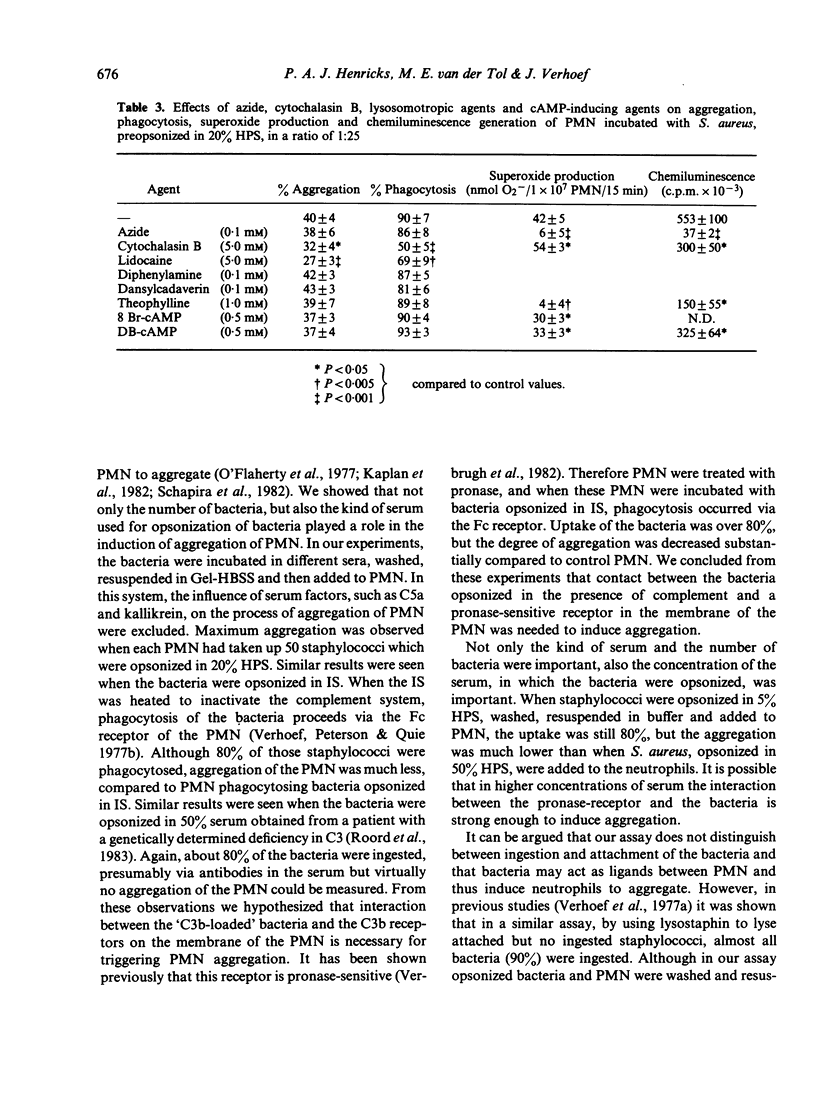
The process of aggregation of human polymorphonuclear leucocytes (PMN) during the uptake of bacteria was studied. Radiolabelled S. aureus were opsonized in different sera, washed, resuspended in buffer and added to the PMN. Uptake of the bacteria and aggregation of the PMN were measured simultaneously. Maximal aggregation occurred within 6 min, when 5 X 10(6) PMN had phagocytosed 2.5 X 10(8) S. aureus. Also the effects of serum concentrations and different sera for opsonization of the bacteria on PMN aggregation were studied. Despite normal uptake, aggregation of PMN was low when bacteria were opsonized in complement-deficient sera. Furthermore when PMN were treated with pronase to inactivate complement receptors on the cell surface of the PMN, and bacteria preopsonized in immune serum were added, no change in uptake occurred, although the degree of aggregation halved compared to control PMN. So, interaction between the bacteria and the complement receptor of the PMN cell membrane is needed for triggering the process of aggregation. By using dansylcadaverin and diphenylamine to modulate lysosomal enzyme release, azide or PMN from a chronic granulomatous disease patient to study the effect of the formation of oxygen species, and theophylline, DB-cAMP or 8 Br-cAMP to increase cAMP levels, it was concluded that aggregation of PMN during phagocytosis was not dependent on oxygen metabolism, degranulation or cAMP levels of PMN.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Fehr J., Dahinden C. Modulating influence of chemotactic factor-induced cell adhesiveness on granulocyte function. J Clin Invest. 1979 Jul;64(1):8–16. doi: 10.1172/JCI109466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Degranulating stimuli decrease the neagative surface charge and increase the adhesiveness of human neutrophils. J Clin Invest. 1980 Feb;65(2):298–306. doi: 10.1172/JCI109672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Bowers T. K., Lammi-Keefe C. J., Jacob H. S., Craddock P. R. Granulocyte aggregometry: a sensitive technique for the detection of C5a and complement activation. Blood. 1980 Jun;55(6):898–902. [PubMed] [Google Scholar]
- Hammerschmidt D. E., Harris P. D., Wayland J. H., Craddock P. R., Jacob H. S. Complement-induced granulocyte aggregation in vivo. Am J Pathol. 1981 Feb;102(2):146–150. [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van der Tol M. E., Thyssen R. M., van Asbeck B. S., Verhoef J. Escherichia coli lipopolysaccharides diminish and enhance cell function of human polymorphonuclear leukocytes. Infect Immun. 1983 Jul;41(1):294–301. doi: 10.1128/iai.41.1.294-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotner G. Z., Lynch J. M., Betz S. J., Henson P. M. Human neutrophil-derived platelet activating factor. J Immunol. 1980 Feb;124(2):676–684. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [PubMed] [Google Scholar]
- O'Flaherty J. T., Ward P. A. Leukocyte aggregation induced by chemotactic factors: a review. Inflammation. 1978 Jun;3(2):177–194. doi: 10.1007/BF00910738. [DOI] [PubMed] [Google Scholar]
- Oseas R., Yang H. H., Baehner R. L., Boxer L. A. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981 May;57(5):939–945. [PubMed] [Google Scholar]
- Quan P. C., Ishizaka T., Bloom B. R. Studies on the mechanism of NK cell lysis. J Immunol. 1982 Apr;128(4):1786–1791. [PubMed] [Google Scholar]
- Ringertz B., Palmblad J., Rådmark O., Malmsten C. Leukotriene-induced neutrophil aggregation in vitro. FEBS Lett. 1982 Oct 18;147(2):180–182. doi: 10.1016/0014-5793(82)81037-5. [DOI] [PubMed] [Google Scholar]
- Rivkin I., Rosenblatt J., Becker E. L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975 Oct;115(4):1126–1134. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Despland E., Scott C. F., Boxer L. A., Colman R. W. Purified human plasma kallikrein aggregates human blood neutrophils. J Clin Invest. 1982 May;69(5):1199–1202. doi: 10.1172/JCI110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke-Grauls C. M., Thijssen R. M., Marcelis J. H., Sharma S. D., Verhoef J. Effects of lysosomotropic amines on human polymorphonuclear leucocyte function. Immunology. 1984 Feb;51(2):319–326. [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Peterson P. K., Verhoef J. Phagocytosis and killing of staphylococci by human polymorphonuclear and mononuclear leucocytes. J Clin Pathol. 1978 Jun;31(6):539–545. doi: 10.1136/jcp.31.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Nguyen B. Y., Sisson S. P., Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982 Oct;129(4):1681–1687. [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., Peters R., van Erne M. E., Daha M. R., Peterson P. K., Verhoef J. Opsonic recognition of staphylococci mediated by cell wall peptidoglycan: antibody-independent activation of human complement and opsonic activity of peptidoglycan antibodies. J Immunol. 1980 Mar;124(3):1167–1173. [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Human polymorphonuclear leucocyte receptors for staphylococcal opsonins. Immunology. 1977 Aug;33(2):231–239. [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]



