Abstract
Deletion of the transforming growth factor β1 (TGF-β1) gene in mice has previously suggested that it regulates both hematopoiesis and angiogenesis. To define the function of TGF-β more precisely, we inactivated the TGF-β type I receptor (TβRI) gene by gene targeting. Mice lacking TβRI die at midgestation, exhibiting severe defects in vascular development of the yolk sac and placenta, and an absence of circulating red blood cells. However, despite obvious anemia in the TβRI–/– yolk sacs, clonogenic assays on yolk sac-derived hematopoietic precursors in vitro revealed that TβRI–/– mice exhibit normal hematopoietic potential compared with wild-type and heterozygous siblings. Endothelial cells derived from TβRI-deficient embryos show enhanced cell proliferation, improper migratory behavior and impaired fibronectin production in vitro, defects that are associated with the vascular defects seen in vivo. We thus demonstrate here that, while TβRI is crucial for the function of TGF-β during vascular development and can not be compensated for by the activin receptor-like kinase-1 (ALK-1), functional hematopoiesis and development of hematopoietic progenitors is not dependent on TGF-β signaling via TβRI.
Keywords: endothelial cell/hematopoiesis/serine/signal transduction/TGF-β/threonine kinase receptor
Introduction
Transforming growth factor β (TGF-β) belongs to a large superfamily of structurally related polypeptides that includes the activins, bone morphogenetic proteins (BMPs) and the growth differentiation factors (GDFs) (Massagué, 1998). TGF-β regulates biological processes as diverse as embryonic development, cell proliferation, differentiation, migration and survival, extracellular matrix (ECM) production and immunomodulation (Roberts and Sporn, 1990; McCartney-Francis and Wahl, 1994). TGF-β is also a very important mediator of vascular development and hematopoiesis, and is generally regarded as having inhibitory effects on endothelial cells and hematopoietic progenitors (Pepper, 1997; Fortunel et al., 2000). However, the actions of TGF-β are complex and largely dependent on the context of individual target cells (Sporn and Roberts, 1988); in most in vitro assays, TGF-β acts as an inhibitor of endothelial proliferation and migration (Muller et al., 1987; Merwin et al., 1991) and a potent stimulant of ECM accumulation (Ignotz et al., 1987).
There are three isoforms of TGF-β in mammals (TGF-β1, 2 and 3). Mice lacking TGF-β1 exhibit two different phenotypes. Approximately 50% of the mice die at midgestation due to defects in yolk sac vasculogenesis and hematopoiesis (Dickson et al., 1995). The other embryos survive beyond birth, possibly through maternal transfer of TGF-β1 (Letterio et al., 1994), but then develop a severe multifocal inflammatory disorder and die at 3–4 weeks of age (Shull et al., 1992; Kulkarni et al., 1993). TGF-β2 null mice have many developmental defects and die perinatally due to congenital cyanosis (Sanford et al., 1997), while TGF-β3 null mice have delayed lung development and die shortly after birth (Kaartinen et al., 1995; Proetzel et al., 1995).
Members of the TGF-β family signal through a heteromeric complex consisting of two types of transmembrane serine/threonine kinases known as type I and type II receptors. Upon ligand binding, type II receptors recruit and transphosphorylate type I receptors, subsequently activating specific intracellular Smad transcription factors (Massagué, 1998). TβRI, also termed activin receptor-like kinase 5 (ALK-5), is a widely expressed type I receptor for TGF-β (Franzén et al., 1993), which can induce the phosphorylation of Smad2 and Smad3. Evidence that TGF-β signaling is of importance for development of the vascular system and hematopoiesis derives from the comparison of mouse embryos lacking either the ligand, TGF-β1 or TβRII. Like TGF-β1 null embryos, TβRII-deficient embryos die at midgestation as a result of defects in yolk sac vasculogenesis and hematopoiesis (Dickson et al., 1995; Oshima et al., 1996). Accumulating data suggest that there is an additional type I receptor for TGF-β in the vascular system. In human umbilical vein endothelial cells, TGF-β has been shown to bind to the endothelial-specific ALK-1 in a heteromeric complex with TβRII (Attisano et al., 1993; ten Dijke et al., 1994; Roelen et al., 1997; Lux et al., 1999; Oh et al., 2000), as well as the more usual TβRI–TβRII complex. In addition, ALK-1-deficient mice die around midgestation, exhibiting severe vascular abnormalities (Oh et al., 2000) reminiscent of mice lacking TGF-β1 or TβRII (Dickson et al., 1995; Oshima et al., 1996). Interestingly, in contrast to TβRI, ALK-1 activates Smad1 and Smad5 (Oh et al., 2000). Thus, TGF-β may signal in endothelial cells through two pathways: one via TβRI, which results in phosphorylation of Smad2 and Smad3, and the other via ALK-1, with the potential to phosphorylate Smad1 and Smad5. In addition to the TGF-β serine/threonine kinase receptors, TGF-β binds to an accessory receptor in endothelial cells, termed endoglin. Interestingly, mutations of endoglin and ALK-1, but not TβRI and TβRII, have been linked to a vascular disorder known as hereditary hemorrhagic telangiectasia (McAllister et al., 1994; Johnson et al., 1996).
In a previous study we sought to examine in more detail the mechanisms through which TGF-β regulates vascular development in the yolk sac (Goumans et al., 1999). By overexpressing either a full-length or a truncated, dominant-negative TβRII in chimeric embryos we demonstrated an important role for TGF-β in maintaining yolk sac integrity (Goumans et al., 1999). Since interfering with TβRII will inhibit all TGF-β signaling, both downstream of TβRI as well as ALK-1, we dissected further the function of TGF-β signaling in yolk sac hematopoiesis and vascular development by generating mice deficient in TβRI. The defects seen in the TGF-β1 and TβRII null embryos suggested a role for TGF-β as an essential regulator of yolk sac hematopoiesis (Dickson et al., 1995; Oshima et al., 1996). We report here that mice lacking TβRI die at around E10.5 due to defects in vascular development of the yolk sac. Surprisingly, the hematopoietic potential was not deficient in these mice; on the contrary, we observed the presence of a normal number of CFU-GM and CFU-mix myeloid progenitor colonies and a large increase in the number of erythroid colonies from mutant yolk sacs compared with controls. Endothelial cells isolated from TβRI-deficient mice exhibited impaired fibronectin production and migration properties that most likely contribute to the abnormal vessel formation. Interestingly, all of the parameters commonly associated with TGF-β signaling and its biological responses were completely blocked in the endothelial cells lacking TβRI, suggesting that TβRI is in fact essential for TGF-β signaling in endothelial cells, despite the presence of ALK-1. Thus, our mouse model has provided important new insights into TGF-β signal transduction and has identified apparently independent mechanisms through which TGF-β regulates vascularization and hematopoiesis during development.
Results
Loss of TβRI results in embryonic lethality
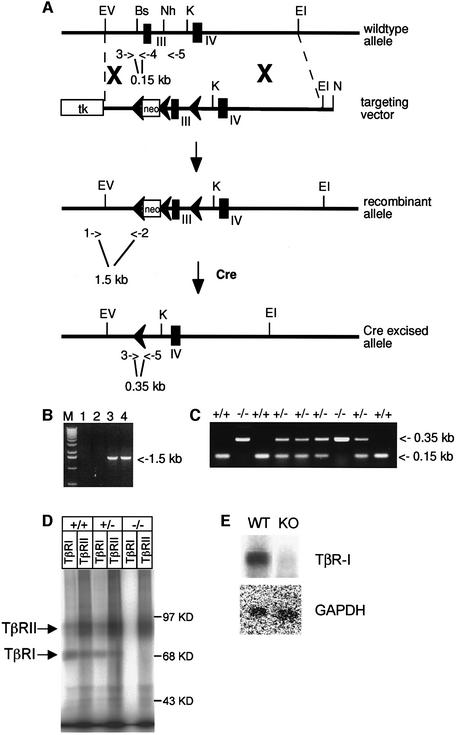
To study the function of TβRI in vivo, a targeting vector for removing exon 3 through Cre/loxP recombination was designed (Figure 1A). Since exon 3 spans the transmembrane domain and the so-called GS domain that is essential for TβRI activation by TβRII (Wieser et al., 1995), it was predicted that deletion of this exon would generate a null allele. After homologous recombination and subsequent Cre transfection, two successfully targeted and Cre-deleted embryonic stem (ES) cell clones were injected into C57BL/6 blastocysts and germline transmission of the null mutation was obtained (Figure 1A–C). Endothelial cells isolated from homozygous mutant embryos were analyzed for receptor expression in a binding assay with [125I]TGF-β1 to confirm that TβRI was absent. Antisera specific for TβRI or TβRII immunoprecipitated a TβRI–TβRII heteromeric complex in the wild-type or heterozygous endothelial cells, but in the homozygous null cells only TβRII was precipitated (Figure 1D). TβRI mRNA levels were determined in homozygous mutant endothelial cells using northern blot analysis. TβRI mRNA was undetectable using a full-length TβRI cDNA probe, indicating that the exon 3 deletion generates an unstable mRNA (Figure 1E). In order to determine whether very low levels of mutated TβRI mRNA might generate a soluble TβRI receptor that could exert a dominant-negative effect, conditioned medium from endothelial cells derived from homozygous mutant embryos was tested for interference with TGF-β signaling in a sensitive TGF-β transcriptional read-out assay (Dennler et al., 1998). Conditioned medium (5 days) from the mutant endothelial cells had no effect on the induction of the reporter gene by TGF-β in HepG2 cells (data not shown). Thus, the possibility of a dominant-negative effect in the mouse through the TβRI mutant allele appears very unlikely.
Fig. 1. Targeting of the TβRI gene. (A) The TβRI wild-type locus, the targeting vector, the targeted allele and the Cre-loxP recombined allele. The targeting vector was generated by inserting a loxP-flanked neomycin (neo) cassette in the Bsu36 site upstream of exon 3 and a single loxP in the NheI site downstream. A thymidine kinase (tk) gene was placed at one end of the construct for negative selection against random integration. Transient expression of the Cre enzyme allowed excision of exon 3 and the neo gene in the targeted allele. Exons are indicated by filled boxes and loxP sites by filled arrows. PCR primers for screening are indicated by open arrows and numbers: 1, RI5′; 2, loxdown; 3, lnl3′; 4, lnl5′; 5, llox3′. Restriction enzymes: Bs, Bsu36; EI, EcoRI; EV, EcoRV; K, KpnI; N, NotI; Nh, NheI. (B) PCR screening for homologous recombination of the targeting vector using the 5′ external primer RI5′ (1) and the vector-specific primer loxdown (2). (C) Genotyping by PCR of an E9.5 litter from a heterozygous intercrossing. The three primers lnl3′ (3), lnl5′ (4) and llox3′ (5) generate specific bands for the wild-type (0.15 kb, primers 3 and 4) and the deleted alleles (0.35 kb, primers 3 and 5). (D) Analysis of TGF-β receptor expression on endothelial cells. Endothelial cells, isolated from wild-type, heterozygous or TβRI–/– embryos were grown to confluency, affinity labeled with [125I]TGF-β1, followed by cross-linking. Cell lysates were subjected to immunoprecipitation using antisera against TβRI or TβRII. (E) Northern blot analysis of total RNA from endothelial cells derived from wild-type and homozygous mutated embryos hybridized with a full-length TβRI cDNA probe. GAPDH mRNA was measured to control for equal loading.
Mice heterozygous for the mutated TβRI allele (TβRI+/–) were fertile and appeared phenotypically normal during development and beyond, also supporting the view that the exon 3 deletion does not generate a soluble TβRI receptor that could exert a dominant-negative effect. Genotyping offspring from heterozygous intercrosses showed only heterozygous and wild-type pups, indicating that TβRI homozygous mutants died during embryogenesis. To determine when death had taken place, embryos from heterozygous intercrosses were analyzed at different gestational stages. At E8.5, homozygous embryos were indistinguishable from their littermates. At E9.5, all TβRI mutants were alive, but had developed severe abnormalities. A majority of the mutant embryos recovered by E10.5 were dead and no live homozygous mutants were recovered after E11.5 (Table I), suggesting that TβRI–/– embryos die at around E10.5.
Table I. Genotype analysis of embryos isolated from TβRI+/– intercrosses.
| Age | +/+ | +/– | –/– | Total |
|---|---|---|---|---|
| E8.5 | 7 (7) | 13 (13) | 7 (7) | 27 |
| E9.5 | 29 (28) | 55 (55) | 22 (22) | 77 |
| E10.5 | 8 (8) | 14 (14) | 7 (2) | 29 |
| E11.5 | 9 (9) | 20 (20) | 8 (0) | 37 |
| Post-natal | 60 | 136 | 0 | 196 |
Numbers in parentheses indicate live embryos.
TβRI–/– embryos have abnormal vascular development
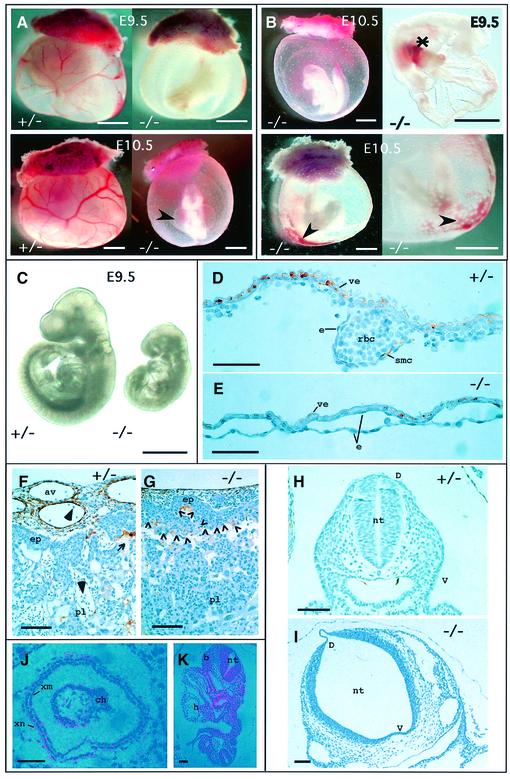
At E9.5, the yolk sacs of TβRI–/– embryos were markedly anemic and lacked a distinct branching network of vessels. This became even more pronounced by E10.5 (Figure 2A). Some embryos exhibited areas of accumulated red blood cells in the yolk sac and hemorrhagic spots on the amnion, indicating leakiness or dilation of the vitelline vessels (Figure 2B). We also detected accumulated blood cells in the vitelline vessels connecting the yolk sac and the embryo proper, suggesting that circulation would be impaired. A circulation defect, causing elevated intracardial pressure, would also explain the pericardial effusion observed in most mutants (Figure 2A). All TβRI–/– embryos were developmentally retarded compared with their littermates at E9.5. They were reduced in size, rarely developing beyond 20 somites, and some embryos had not turned or were still in the process of turning (Figure 2B and C). Histological sections of wild-type and mutant yolk sacs revealed that vessel-like structures were indeed formed in mutants, but they appeared enlarged and fragile and contained few red blood cells compared with controls (Figure 2D and E). Staining E9.5 sections with anti-smooth muscle cell actin antibody showed that smooth muscle cells had started to surround the endothelial-lined vessels of the yolk sac in wild-type and TβRI+/– embryos (Figure 2D), while this was clearly not the case in the TβRI–/– mutants at this stage (Figure 2E), or even 1 day later (not shown). These results are compatible with expression of TβRI mRNA at E7.5, the time at which or just before the precursors of endothelial and hematopoietic cells develop (Figure 2J), as well as in the yolk sac mesoderm 1 day later (Goumans et al., 1999). Sections through the placenta demonstrated a defect in vascularization in TβRI–/– embryos, with embryonic vessels apparently unable to sprout properly into the labyrinthine layer (Figure 2G). The vasculature derived from the extra-embryonic tissue of controls had clearly infiltrated the maternal tissue at E9.5 (Figure 2F), the allantoic vessels were dilated and surrounded by smooth muscle cells (Figure 2F); this allows intermingling of fetal and maternal blood cells. Both endothelial cells and smooth muscle cells of the placenta express TβRI (data not shown). Allantoic vessels in the placentae of the TβRI–/– embryos, on the other hand, were significantly smaller, and smooth muscle cells did not invade the deeper-lying labyrinthine layers through the ectoplacental plate (open arrowheads in Figure 2G), which appeared to act as a barrier precluding mixing of fetal and maternal blood. Intermingling of embryonic and maternal vessels in this layer is necessary for proper exchange of nutrients between the two circulations, but is probably not the cause of death since embryos are not dependent on placental exchange at E9.5 (Cross et al., 1994) when the mutants already have severe defects. These defects included abnormalities in the neural tube, which was kinked (not shown) and, in histological sections, dilated and thin (Figure 2H and I), particularly ventrally. Again, in situ hybridization showed that TβRI is expressed in the neural tube at E8.5 (Figure 2K), just before the phenotype becomes evident, and is coincident ventrally with expression of TβRII, described previously (Wang et al., 1995). This makes it likely that the neural tube defects are a direct result of the lack of TβRI expression in cells of the neural tube and are not an indirect effect, secondary to impaired yolk sac circulation. Impairment of circulation at this stage can result in hypoxia causing dilation of internal cavities in the embryo (Iyer et al., 1998), including that of the neural tube and heart sac, somewhat similar to that observed here.
Fig. 2. TβRI–/– embryos exhibit severe defects in vascular development. (A) Gross morphology of whole-mount yolk sacs in mutant embryos compared with heterozygous littermates. Note the enlarged pericardial sac (arrowhead). (B) Defects in TβRI–/– embryos. Some embryos have not turned (upper left). Asterisk, accumulated blood in vitilline vessels connecting the yolk sac and the embryo proper. Arrowheads, leakage of red blood cells and dilated vascular structures. (C) By E9.5 mutant embryos are severely growth retarded. (D–G) Immunohistochemical staining for smooth muscle cell actin in transverse sections of mutant embryos compared with heterozygous littermates. (D) Section through the yolk sac of a heterozygote at E9.5. Endothelial cells are either in close apposition with the visceral endoderm or contain red blood cells within the developing vessel. Smooth muscle cells, stained brown, begin to differentiate from mesenchyme and contribute to the structure of the vessel wall. (E) Section through the yolk sac of a mutant at E9.5. The vessels are lined with endothelial cells but are dilated, and have very few red blood cells and no smooth muscle cells. (F) Section through chorioallantoic region of the placenta of a heterozygote at E9.5 showing smooth muscle cells surrounding the dilated allantoic blood vessels derived from extra-embryonic tissue and their invasion of the labyrinthine part of the placenta (maternal part). This allows intermingling of the fetal (large arrowheads) and maternal (small arrow) red blood cells. (G) Allantoic blood vessels in the placenta of a mutant at E9.5 are much smaller, there are fewer smooth muscle cells and there is no evidence for their invasion of the maternal labyrinthine layer through the ectoplacental plate. The limit of ectoplacental plate is indicated by the row of open arrowheads in (G). (H and I) Histological sections of the neural tube at the level of the heart in a heterozygote (H) and homozygote (I) at E8.5. (J) Expression of TβRI at E7.5 in the extra-embryonic part of the conceptus. (K) Expression of TβRI in the rostral neural tube at the level of the heart at E8.5. Abbreviations: av, allantoic blood vessel; b, branchial arch; ch, chorion; D, dorsal; e, endothelial cell; ep, ectoplacental plate; h, heart; nt, neural tube; pl, placenta; rbc, red blood cell; smc, smooth muscle cells; V, ventral; ve, visceral endoderm; xm, extra-embryonic mesoderm; xn, extra-embryonic endoderm. Scale bars, 1 mm (A–C), 50 µm (D–E), 100 µm (F–K).
Taken together, our observations suggest that the impaired yolk sac circulation is the primary cause of death in TβRI–/– embryos. The presence of vessels in the yolk sacs of the mutant embryos demonstrates that the formation of a primary capillary plexus in situ (vasculogenesis) (Risau, 1997) does occur, although the resulting vasculature is defective. However, the lack of vessel sprouting in the placenta indicates that branching from pre-existing vessels (angiogenesis) is inhibited.
Progenitors from TβRI–/– yolk sacs have increased erythroid colony-forming ability
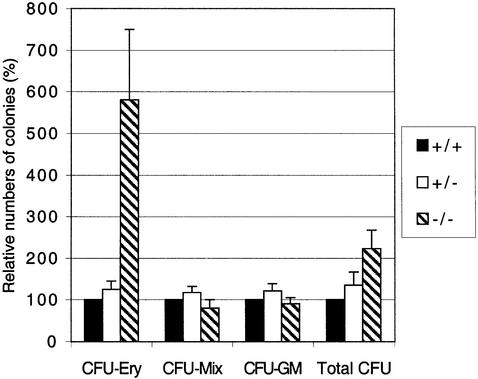
The phenotype of the TβRI–/– embryos is similar to the TGF-β1–/– embryos, where defects in vascular development and hematopoiesis were reported (Dickson et al., 1995). The very low numbers of red blood cells detected in the yolk sac vessels of TβRI–/– embryos (Figure 2E) prompted us to examine further hematopoietic function in these embryos. This is also of interest since TGF-β is thought to play an important role in regulating proliferation of hematopoietic progenitors (Keller et al., 1992; Sitnicka et al., 1996; Fortunel et al., 2000). We therefore determined whether hematopoietic progenitors developed and/or were present in the yolk sacs of mutant embryos. We tested the ability of cell suspensions from mutant and control yolk sacs to form hematopoietic colonies in an in vitro clonogenic assay (Wong et al., 1986). No significant difference was observed in the ability to form myeloid colonies (CFU-GM and CFU-mix), whereas a large increase in the number of pure erythroid colonies (CFU-Ery) was observed in mutants compared with controls (Figure 3). This suggests that the lack of blood cells in TβRI–/– yolk sacs is not due to an intrinsic defect in hematopoiesis, but rather that the yolk sac environment does not permit proper differentiation and maturation of hematopoietic precursors and their progeny.
Fig. 3. Increased erythroid and normal myeloid potential in precursors from TβRI–/– yolk sacs. Yolk sacs were collected from E9.5 embryos, dispersed, and equal numbers of cells plated in methylcellulose supplemented with growth factors. Colonies were scored 6 days later. Data from three different experiments including seven litters and 11 mutant embryos. CFU, colony forming unit; Ery, erythroid; Mix, erythroid-myeloid; GM, myeloid (granulocyte–macrophage).
TβRI mediates TGF-β signaling in endothelial cells
To gain more insight into the mechanism underlying the phenotype, we isolated and immortalized endothelial cells and fibroblasts from embryos of heterozygous crossings. TβRI could not be detected in homozygous mutant endothelial cells (Figure 1D). We used a Smad3/Smad4 binding element (so-called CAGA) coupled to a luciferase reporter as a TGF-β transcriptional read-out assay (Dennler et al., 1998). As shown in Figure 4A, activation of this reporter is dependent on the presence of at least one functional TβRI allele in both fibroblasts and endothelial cells. The transcriptional activation of the CAGA-reporter was completely restored by re-introducing the TβRI in the knockout endothelial cells. Consistent with the lack of transcriptional activation upon TGF-β stimulation due to lack of Smad phosphorylation by the TβRI, a band of phosphorylated Smad2 in TGF-β-stimulated wild-type endothelial cells was not observed in homozygous mutant cells (Figure 4B). Thus, all signaling responses measured here were completely blocked in TβRI null endothelial cells.
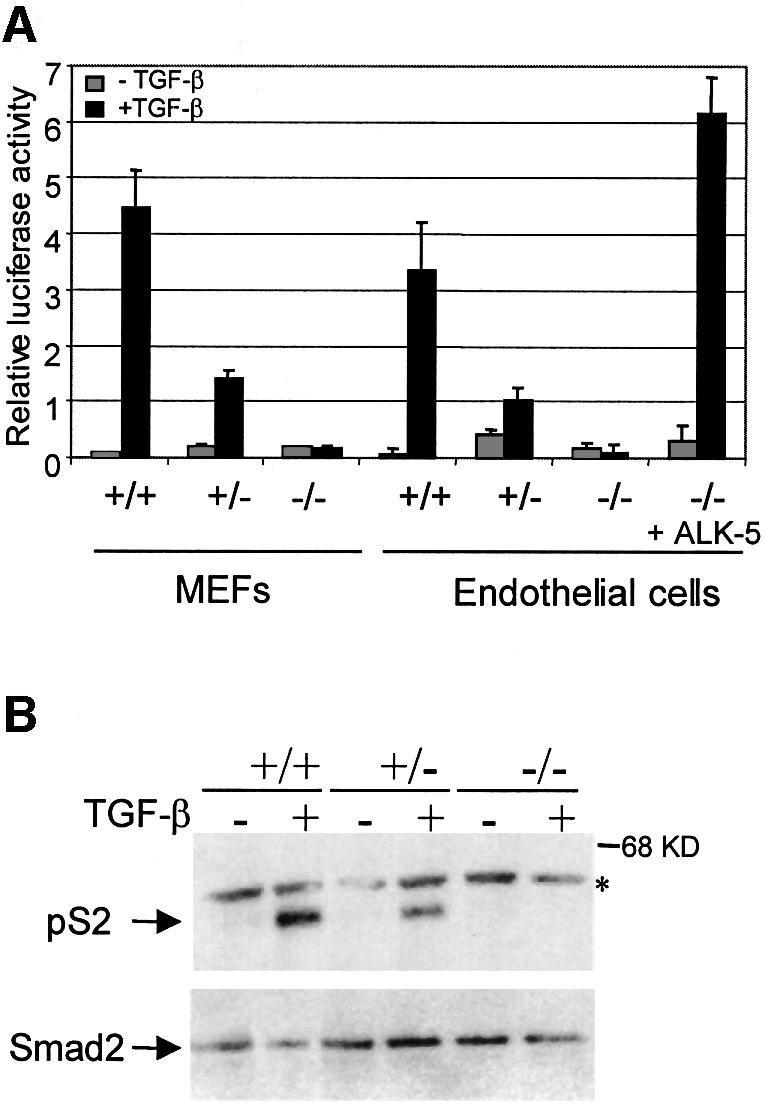
Fig. 4. TβRI mediates TGF-β signaling in endothelial cells. (A) TGF-β-induced transcriptional activation of (CAGA)12 luciferase reporter is impaired in TβRI mutant cells. Cells were transfected and stimulated for 16 h without (light) or with (dark) TGF-β1, followed by the measurement of the luciferase activity in the cell lysates. In the case of the TβRI-deficient endothelial cells, an expression plasmid for TβRI was co-transfected (+ALK-5) to demonstrate that introduction of exogenous TβRI is sufficient to restore the TGF-β-induced transcriptional activity. (B) TGF-β-induced Smad phosphorylation is impaired in TβRI–/– endothelial cells. Endothelial cells, isolated from either TβRI knockout embryos or wild-type littermates, were serum starved and stimulated with TGF-β1 for 1 h. Samples were transferred and incubated with an antiserum against phosphorylated Smad2 (PS-2). Equal levels of Smad2 protein in each lane are shown. The asterisk indicates a non-specific band.
No TGF-β induced fibronectin production in TβRI–/– endothelial cells
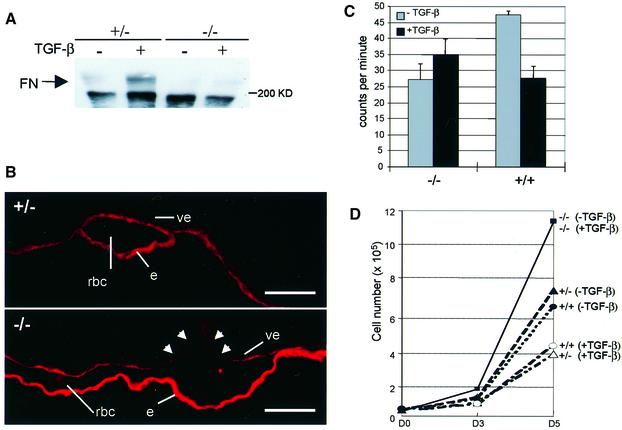
One important target gene for TGF-β is fibronectin, an ECM molecule that is present in large amounts between the visceral endoderm and extra-embryonic mesoderm layers of the visceral yolk sac. The ECM components are critical for stabilizing the developing vasculature. The composition of the ECM also plays an important role in the regulation of endothelial precursors, and determines whether these cells will proliferate and migrate or whether they will differentiate and form a vascular network (Baldwin, 1996). We therefore determined the capacity of the endothelial cells to produce fibronectin. Upon TGF-β stimulation, fibronectin production was enhanced in the heterozygous cell line but no increase was seen in the TβRI–/– endothelial cells (Figure 5A). Histological sections of embryos were stained with an anti-fibronectin antibody to determine whether fibronectin production was reduced in vivo. Figure 5B indeed shows a reduction in fibronectin deposited in the yolk sac in a representative section through a TβRI–/– embryo, compared with that in a heterozygote. This indicates that there is reduced fibronectin production in the mutant embryos, which could affect vessel formation both at the level of vasculogenesis, by reduced ECM support to the vascular plexus, and at the level of angiogenesis, through dysregulation of endothelial precursors. These results also suggest a crucial role for TβRI in TGF-β-mediated regulation of ECM deposition by endothelial cells in the visceral yolk sac.
Fig. 5. No TGF-β-mediated fibronectin synthesis and growth inhibition in TβRI–/– endothelial cells. (A) Endothelial cells were serum starved and stimulated with TGF-β1. Samples were electrophoresed, transferred and incubated with an anti-fibronectin antibody (FN). (B) Representative histological sections of yolk sacs from heterozygous (+/–, top panel) and TβRI–/– (–/–, bottom panel) embryos were stained with an anti-fibronectin antibody. Arrowheads indicate discontinuities in fibronectin deposition under the visceral endoderm around the vessels in the mutant embryo. Abbreviations (e, rbc and ve) are as in the legend to Figure 2. The scale bar is 50 µm. (C) Endothelial cells were treated with 5 ng/ml TGF-β1 for 24 h and [3H]thymidine was added for an additional 4 h to monitor DNA synthesis. The figure shows the mean [3H]thymidine incorporation of triplicate samples. (D) Cells were seeded in the absence or presence of TGF-β1. On day 3 and day 5 the total cell number per well was counted. Shown is a representative experiment giving the mean of triplicate samples.
Endothelial cells lacking TβRI are insensitive to TGF-β-mediated growth inhibition
In order to characterize the growth inhibitory response by TGF-β in TβRI-deficient endothelial cells, these cells were cultured in the presence or absence of TGF-β. TGF-β had no effect on the thymidine incorporation rate and total cell numbers determined after 3 and 5 days; however, wild-type and heterozygous endothelial cells were clearly growth inhibited by TGF-β (Figure 5C and D). This demonstrates that the functional block in TGF-β signaling in the TβRI–/– endothelial cells extends to growth inhibition. Importantly, we also observed an overall increase in growth rate for the mutant cells compared with controls, in the absence of exogenous TGF-β (Figure 5C and D). Should a similar increase in growth rate or a lack of growth inhibition occur in vivo, it could contribute to the phenotype observed in the mutant yolk sacs.
Impaired migration of TβRI-deficient endothelial cells
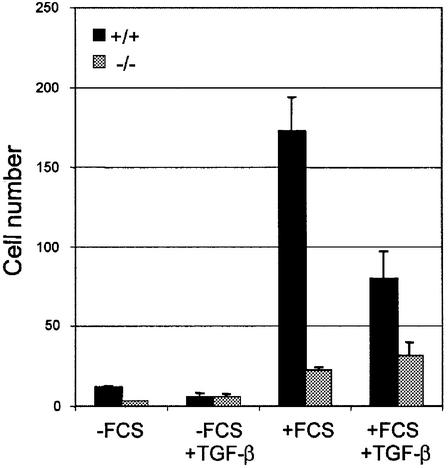
To assess whether the impaired angiogenesis might be the result of altered migration of endothelial cells we conducted a migration assay with the isolated endothelial cells. While wild-type cells migrate on fibronectin in the presence of serum, surprisingly TβRI–/– endothelial cells show virtually no enhancement of migration upon serum addition (Figure 6). This increase in migration of wild-type endothelial cells was inhibited by TGF-β addition. Mutant endothelial cells, on the other hand, did not change their migratory behavior upon TGF-β stimulation. The TβRI–/– endothelial cells are able to form tubes in a 3D-collagen gel and addition of TGF-β has no effect on their behavior, indicating that they have no intrinsic defect in tube formation in vitro (data not shown). This suggests that TGF-β signaling is essential for proper migration of endothelial cells and, together with a reduced fibronectin production, provides an explanation for the defective angiogenesis in TβRI–/– embryos.
Fig. 6. Impaired migration of TβRI–/– endothelial cells. The migration assay was performed using a Boyden chamber and an 8 µm filter. Cells were added to the upper wells with or without FCS, and with or without TGF-β1. After 5 h, cells that had migrated to the lower wells were counted. The figure shows a representative experiment. Values are expressed as means of four wells.
Discussion
TGF-β is generally believed to be a key player in the regulation of hematopoiesis and vascular development (Dickson et al., 1995; Pepper, 1997; Fortunel et al., 2000). However, the precise mechanisms of how TGF-β exerts its effects have remained largely unknown. Here we have analyzed the functional properties of endothelial cells and hematopoietic progenitors from TβRI-deficient mice to investigate how TGF-β signaling regulates vascular development and the growth and differentiation of hematopoietic progenitors. TβRI–/– embryos die between E10 and E11, exhibiting pale and anemic yolk sacs that lack a proper vascular network. Histological examination of the yolk sacs showed malformed vessels containing few blood cells, very similar to the phenotypes observed in the TGF-β1- and TβRII-deficient embryos (Dickson et al., 1995; Oshima et al., 1996). While few red blood cells were detected in the TβRI–/– embryos, the number of erythroid colonies was increased and other myeloid colonies were normal. Endothelial cells from mutant embryos exhibited several defects including impaired migration and fibronectin synthesis. These defects are almost certainly due to a TβRI null mutation, since the tissues prominently affected by the mutation (the yolk sac, placenta and neural tube) all express TβRI at the time, or just before, the phenotype becomes evident. In addition, the TβRI protein could not be immunoprecipitated from mutant endothelial cells, and mutant TβRI mRNA could not be detected by northern blot analysis. Conditioned medium from the TβRI–/– endothelial cells did not block TGF-β signaling in a sensitive transcriptional read-out assay and the heterozygous embryos were indistinguishable from wild-type embryos. These data, together with previously observed biochemical properties of TβRI, i.e. that TβRI alone can not bind ligand (Laiho et al., 1991; Wrana et al., 1994), rule out the possibility of a dominant-negative effect created by the engineered mutation.
In addition to a TβRI–TβRII receptor complex, endothelial cells express other potential signaling partners such as the accessory receptor endoglin, and ALK-1 and its downstream effector Smad5. Mice lacking any of these genes exhibit similar defects in the yolk sac: endothelial cell differentiation and vasculogenesis is normal until E8.5, dilated yolk sac vessels are evident at E9.5 and the phenotype is lethal at around E10.5–11.5, in all cases accompanied by severe growth retardation (Chang et al., 1999; Li et al., 1999; Yang et al., 1999; Oh et al., 2000). In addition to these vascular defects in the yolk sac, Smad5-, ALK-1- and endoglin-deficient embryos have defects in the developing amnion, gut and heart, and in many Smad5 mutants the allantois is fused to the chorion and is not well elongated (Goumans and Mummery, 2000). It is surprising that the other TGF-β type I receptor (ALK-1), expressed prominently in endothelial cells, can not rescue the vascular phenotype in the TβRI–/– embryos. One possible explanation for this lack of redundancy with ALK-1 could be that TβRI and ALK-1 mediate TGF-β signaling through different intracellular pathways as suggested by Oh et al. (2000). However, all signaling responses to TGF-β that we measured, including ALK-1-mediated responses, were completely blocked in the TβRI–/– endothelial cells, despite the expression of ALK-1 (M.-J.Goumans and P.ten Dijke, manuscript in preparation). It is therefore possible that TβRI is required for all TGF-β signaling responses in endothelial cells.
The vascular defects in the mutant embryos fall broadly into two categories. One is that of a distended and fragile primary vascular plexus in the yolk sac. The other is that of an apparent absence of angiogenesis. Interestingly, TβRI–/– endothelial cells develop normally and are capable of fusing and forming a lumen, as shown both by the presence of a primary vascular plexus in the yolk sac and intact tube-forming properties in vitro. There must, therefore, be other mechanisms behind the vascular defects. We analyzed the function of TβRI–/– endothelial cells in detail and discovered other alterations in the properties of these cells that could provide mechanistic explanations for both types of defects. First, an overall increase in growth rate or lack of TGF-β-induced growth inhibition of endothelial cells could contribute to the distension of vessels during vascular development. Secondly, reduced fibronectin production and, therefore, inadequate distribution of ECM components destabilizes the yolk sac layers, which then can not support the vascular plexus properly. This probably leads to dilated and leaky vessels as observed upon both gross and histological examination of mutant yolk sacs. Altered composition of the ECM might also cause dysregulation of endothelial precursors (Baldwin, 1996), which could contribute to the failure of angiogenesis. Thirdly, impaired migration of the endothelial cells is probably the main reason for the absence of angiogenesis since chemotactic migration of endothelial cells is a critical event in angiogenesis (Risau, 1997). Finally, some vessels may lack vascular smooth muscle cells due to a defect in the capacity of mesenchymal cells to differentiate into smooth muscle cells in response to TGF-β. We propose that the altered properties of TβRI–/– endothelial cells described here are sufficient to explain the lethal phenotype of the mutant embryos, without the neural tube defects playing a significant role. Endothelial-specific inactivation of the TβRI gene would confirm this.
To date, the analysis of hematopoiesis in the TGF-β1 knockout mice has been based on in situ detection of embryonic globin in the yolk sacs. Very few red blood cells were detected and this led to the interpretation that TGF-β is an essential positive regulator of primitive yolk sac hematopoiesis (Dickson et al., 1995). Here we used a different approach and assayed hematopoietic precursors from yolk sacs in vitro. The large number of erythroid colonies and the normal number of CFU-GM and CFU-mix colonies in the TβRI mutants contrast strikingly with the very anemic appearance of these embryos. We believe that there is no intrinsic defect in hematopoiesis, but rather that the environment in the yolk sac, with malformed vascular structures, does not support proper hematopoiesis. In this context, it is of interest that post-natal hematopoiesis seems to be normal in the fraction (50%) of TGF-β1 null mice that survive beyond birth, possibly through transfer of maternal TGF-β1 (Shull et al., 1992; Kulkarni et al., 1993; Letterio et al., 1994). In addition, engraftment and repopulation of all hematopoietic lineages was observed in transplanted recipients where the donor bone marrow was derived from TGF-β1 null mice (Yaswen et al., 1996). Therefore, it seems that there is normal hematopoietic stem cell function post-natally in TGF-β1 null mice, and the results of the present study show that normal myeloid progenitors are generated in mice deficient in TGF-β signaling during development. The very high number of erythroid colonies could be explained by an accumulation of progenitors resulting from impaired differentiation in the yolk sac. However, it is more likely that the lack of TGF-β inhibition increases the erythroid colony-forming potential of individual cells in vitro. This has been demonstrated for adult bone marrow-derived progenitors when assaying erythroid colonies in the presence of neutralizing antibodies to TGF-β (Dybedal and Jacobsen, 1995). The inhibitory effects of TGF-β in hematopoiesis have mainly been described otherwise for very immature precursors and stem cells (Sitnicka et al., 1996), while more committed myeloid precursors are less inhibited or, at certain stages, even stimulated (Keller et al., 1992). This is probably the reason why we saw little difference in the numbers of CFU-GM and CFU-mix colonies. It will be interesting to study the function of the more primitive stem cells residing in the embryo proper, in the so-called para-aortic splanchnopleura (P-Sp) (Cumano et al., 1996), and to see how the lack of TGF-β signaling influences the proliferative and developmental potential of these cells.
In summary, our results suggest that TβRI is an essential type I receptor for TGF-β in endothelial cells, and that TGF-β signaling is necessary for normal vascular development in regulating the proliferation, extracellular matrix production and migration of endothelial cells. Addition ally, and in contrast to previous reports, we show that TGF-β signaling is not crucial for the growth and differentiation of hematopoietic progenitors during development.
Materials and methods
Targeting of the TβRI genomic locus
A 129/Sv Lambda FIX II genomic phage library was screened with a 31 bp oligonucleotide probe (CAGTGTTCTGGGAAATTGCTCGACGCTGTTTC) from the intracellular part of TβRI. An 18 kb clone was obtained and two KpnI fragments were subcloned in pBluescript SKII (pSKII), rendering one 8 kb clone containing exon 3 and one 7 kb clone with exons 4 and 5. A single loxP site was isolated as a 90 bp EcoRI–PstI fragment from pGZM (gift from H.Gu) and ligated into the NheI site downstream of exon 3. A 1.3 kb SalI–XbaI fragment containing a loxP-flanked neo gene under the HSV-tk promoter from pl2neo (H.Gu) was inserted into the Bsu36 site upstream of exon 3. For negative selection, a thymidine kinase gene under the PGK promoter was isolated from pPNT (R.Jaenisch) as an EcoRI–HindIII fragment and inserted into the XbaI site of the vector pVZ-I, generating pVZ-Itk. The final construct was assembled by semi-blunt ligations of an EcoRV–KpnI fragment of the modified 5′ genomic clone and a KpnI–EcoRI fragment of the 3′ clone between the XbaI and BamHI sites in pVZ-Itk.
The TβRI targeting construct was linearized with NotI and electroporated into two ES cell lines, the RI and Genome Systems (GS). Cells were grown under selection (300 µg/ml G418 and 4 µM gancyclovir). Colonies were screened for homologous recombination by PCR using the 5′ external primer RI5′ (CTCAGCAGAGGAATGGATACAGAAAATG) and the vector-specific primer loxdown (ATTAAGGGTTATTGAATATGATCGG). Co-integration of the third loxP site was verified by PCR using the primers loxdown and loxup (CGAAGTTATTAGGTCTGAAGAGGAG), which amplify sequences between loxP sites. Correctly targeted clones were transiently transfected with pIC-Cre, subcloned, and neomycin-sensitive clones were analyzed by PCR for Cre/loxP recombination events using the loxdown and loxup primers. Two separate clones carrying a deletion of exon 3, derived from either of the two ES cell lines, were then injected into C57BL/6 blastocysts. Chimeric mice were obtained and mated for germline transmission. Screening of mice and embryos was carried out with a three primer PCR strategy: lnl5′ (ATGAGTTATTAGAAGTTGTTT), lnl3′ (ACCCTCTCACTCTTCCTGAGT) and llox3′ (GGAACTGGGAAAGGAGATAAC).
Immunohistochemistry
Litters from heterozygous crossings were collected at E8.5–10.5 and processed in the yolk sac after removal of the decidua. Embryos were collected in cold phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde overnight, dehydrated, embedded in paraffin and sectioned (7 µm). Immunohistochemistry was carried out as described previously (Zwijsen et al., 1999) with the exception that incubation with anti-smooth muscle actin antibody (α-SMCA), diluted 1:150 (Sigma, Zwijndrecht, The Netherlands), was overnight at 4°C. Secondary antibody was biotinylated goat anti-mouse IgG (dilution 1:250; Amersham, Roosendaal, The Netherlands). An enhancement step with Avidin-Biotin Complex (DAKO) was performed according to the manufacturer’s instructions. The sections were counterstained with hematoxylin and mounted in DEPEX (Merck, Darmstadt, Germany). Immunofluorescent staining for fibronectin was performed as decribed in Goumans et al. (1999).
Probe synthesis and in situ hybridization
Two TβRI-specific oligonucleotide primer pairs were designed to amplify a 443 bp and a 311 bp region corresponding to bp 1368–1811 and bp 2009–2320 of TβRI (Suzuki et al., 1994) from mouse embryonic heart cDNA using PCR. The cDNA was ligated in pBS SKII– and the correct nature of the fragment was verified by sequencing. In situ hybridization was performed as described previously (Feijen et al., 1994).
Hematopoietic clonogenic progenitor assay
Yolk sacs from heterozygous intercrosses were collected at E9.5 and disaggregated by drawing them through a 26G needle. DNA was isolated from the remnants of the embryos, and genotyping of each embryo was determined by PCR as described above. Five thousand cells from each yolk sac were plated in duplicate in methylcellulose matrix containing interleukin (IL)-3 (10 ng/ml), IL-6 (10 ng/ml), stem cell factor (50 ng/ml) (Myelocult 3534 Stem Cell Technologies, Inc., Vancouver, Canada) and erythropoeitin at 3 U/ml. After 6 days in culture (37°C/5% CO2) colonies were scored under the microscope.
Generation and culture of endothelial and fibroblast cell lines
Embryos from heterozygous intercrossings were collected at E9.5, disaggregated enzymatically in trypsin (10 min at room temperature) and plated. For selective transformation of endothelial cells, the embryonic cells were infected with a retrovirus expressing the Polyoma middle-T oncogene (Williams et al., 1988) and then grown for a sufficient number of passages so that cells with an endothelial morphology became evident. For generation of fibroblast cell lines, embryonic cells were plated and infected with a retroviral vector containing the SV40 large-T oncogene (Jat and Sharp, 1986). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), non-essential amino acids, l-glutamine and penicillin/streptomycin on 0.1% gelatin-coated dishes. Cells were grown in 5% CO2-containing atmosphere at 37°C.
Affinity cross-linking and immunoprecipitations
TGF-β1 was iodinated by the chloramine-T method (Frolik et al., 1984). Affinity binding and cross-linking of iodinated TGF-β to its receptors, followed by immunoprecipitation with receptor-specific antibodies (α-TβRI, Franzén et al., 1993; α-TβRII, Yamashita et al., 1994) was performed as described by Piek et al. (1999).
Western blot analysis
Mouse endothelial cells were grown to 90% confluence, serum starved in 0.5% FBS-containing medium for 16 h, followed by 1 h stimulation with 10 ng/ml TGF-β1 for Smad2 phosphorylation and 6 h for fibronectin deposition. Western blot analysis was performed as described by Persson et al. (1998). Primary antibodies against phosphorylated Smad2 (Persson et al., 1998) and fibronectin (Sigma) were diluted 1000-fold in TBS-T, and secondary horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Amersham) was used in a 10 000-fold dilution in TBS-T. Detection was by ECL.
Transient transfection and transcriptional reporter assays
Cells were seeded at a density of 2 × 105 cells per well in 6-well plates. The next day, cells were transfected with 0.5 µg of the TGF-β-inducible (CAGA)12 luciferase reporter (Dennler et al., 1998) with or without an expression plasmid encoding TβRI. Transfection was carried out using the calcium phosphate co-precipitation method (fibroblasts) or Fugene 6 transfection reagent (endothelial cells) following the manufacturer’s protocol (Boehringer Mannheim). After 24 h, cells were stimulated with 10 ng/ml TGF-β1 for 16 h, lysed with Reporter Lysis Buffer (Promega), followed by measurement of luciferase activity and β-galactosidase activity as described by Piek et al. (1999). The experiments were performed in triplicate at least three times; representative experiments are shown.
DNA synthesis by thymidine incorporation assay
Endothelial cells were seeded in DMEM containing 5% FBS at a density of 5 × 104 cells per well, in a 24-well culture plate. The next day, TGF-β1 was added at 5 ng/ml. After 24 h, 1 µCi/ml [3H]thymidine was added for an additional 4 h. [3H]thymidine was measured as described by Piek et al. (1999). The experiments were performed at least twice, and a representative experiment showing the mean and standard deviation of triplicate observations is presented in Figure 5.
Migration assay
Migration (chemokinesis) was measured using a Boyden chamber. A Costar nucleopore filter (8 µm pore) was coated with fibronectin overnight at 4°C. The chamber was washed with PBS and the lower chamber was filled with DMEM with or without serum and with or without TGF-β1 (10 ng/ml). Cells were trypsinized and suspended at a final concentration of 50 000 cells/ml in DMEM. Cell suspension (150 µl) was added into the upper chamber and incubated at 37°C. After 5 h, cells were washed and the upper surface was wiped to remove the non-migrating cells. The membranes were fixed in methanol, washed with water, stained, and the number of cells present on the lower surface was counted.
Acknowledgments
Acknowledgements
We would like to thank Kristina Sundgren and Lilian Wittmann for help with animal husbandry, Jeroen Korving for sectioning embryos, Dr Carl-Henrik Heldin for valuable discussion and comments on the manuscript, Drs Reinhard Fässler and Cord Brakebusch for helpful discussions and generously sharing reagents, and Dr Sten Eirik Jacobsen for advice on hematopoietic progenitor assays. This work was supported by grants from Cancerfonden, The ALF Clinical Research Grant Program from Lund University Hospital and Astra Draco AB, Lund (presently Astra-Zeneca) to S.K., and grants from The Netherlands Heart Foundation (grant 99-046) and Life Science Foundation (ALW 809.67.020 and 809.67.021) to P.t.D. and C.L.M.
References
- Attisano L., Carcamo,J., Ventura,F., Weis,F.M., Massagué,J. and Wrana,J.L. (1993) Identification of human activin and TGF-β type I receptors that form heteromeric kinase complexes with type II receptors. Cell, 75, 671–680. [DOI] [PubMed] [Google Scholar]
- Baldwin H.S. (1996) Early embryonic vascular development. Cardiovasc. Res., 31, E34–E45. [PubMed] [Google Scholar]
- Chang H., Huylebroeck,D., Verschueren,K., Guo,Q., Matzuk,M.M. and Zwijsen,A. (1999) Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development, 126, 1631–1642. [DOI] [PubMed] [Google Scholar]
- Cross J.C., Werb,Z. and Fisher,S.J. (1994) Implantation and the placenta: key pieces of the development puzzle. Science, 266, 1508–1518. [DOI] [PubMed] [Google Scholar]
- Cumano A., Dieterlen-Lievre,F. and Godin,I. (1996) Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell, 86, 907–916. [DOI] [PubMed] [Google Scholar]
- Dennler S., Itoh,S., Vivien,D., ten Dijke,P., Huet,S. and Gauthier,J.-M. (1998) Direct binding of Smad3 and Smad4 to critical TGF-β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J., 17, 3091–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson M.C., Martin,J.S., Cousins,F.M., Kulkarni,A.B., Karlsson,S. and Akhurst,R.J. (1995) Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development, 121, 1845–1854. [DOI] [PubMed] [Google Scholar]
- Dybedal I. and Jacobsen,S.E. (1995) Transforming growth factor-β (TGF-β), a potent inhibitor of erythropoiesis: neutralizing TGF-β antibodies show erythropoietin as a potent stimulator of murine burst-forming unit erythroid colony formation in the absence of a burst-promoting activity. Blood, 86, 949–957. [PubMed] [Google Scholar]
- Feijen A., Goumans,M.-J. and van den Eijnden-van Raaij,A.J. (1994) Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development, 120, 3621–3637. [DOI] [PubMed] [Google Scholar]
- Fortunel N.O., Hatzfeld A. and Hatzfeld,J.A. (2000) Transforming growth factor-β: pleiotropic role in the regulation of hematopoiesis. Blood, 96, 2022–2036. [PubMed] [Google Scholar]
- Franzén P., ten Dijke,P., Ichijo,H., Yamashita,H., Schulz,P., Heldin,C.-H. and Miyazono,K. (1993) Cloning of a TGF-β type I receptor that forms a heteromeric complex with the TGF-β type II receptor. Cell, 75, 681–692. [DOI] [PubMed] [Google Scholar]
- Frolik C.A., Roller,P.P., Cone,J.L., Dart,L.L., Smith,D.M. and Sporn,M.B. (1984) Inhibition of transforming growth factor-induced cell growth in soft agar by oxidized polyamines. Arch. Biochem. Biophys., 230, 93–102. [DOI] [PubMed] [Google Scholar]
- Goumans M.-J. and Mummery,C. (2000) Functional analysis of the TGF-β receptor/Smad pathway through gene ablation in mice. Int. J. Dev. Biol., 3, 253–265. [PubMed] [Google Scholar]
- Goumans M.-J., Zwijsen,A., van Rooijen,M.A., Huylebroeck,D., Roelen,B.A. and Mummery,C.L. (1999) Transforming growth factor-β signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development, 126, 3473–3483. [DOI] [PubMed] [Google Scholar]
- Ignotz R.A., Endo,T. and Massagué,J. (1987) Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-β. J. Biol. Chem., 262, 6443–6446. [PubMed] [Google Scholar]
- Iyer N.V. et al. (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1. Genes Dev., 12, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P.S. and Sharp,P.A. (1986) Large T antigens of simian virus 40 and polyomavirus efficiently establish primary fibroblasts. J. Virol., 59, 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.W. et al. (1996) Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nature Genet., 13, 189–195. [DOI] [PubMed] [Google Scholar]
- Kaartinen V., Voncken,J.W., Shuler,C., Warburton,D., Bu,D., Heisterkamp,N. and Groffen,J. (1995) Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial–mesenchymal interaction. Nature Genet., 11, 415–421. [DOI] [PubMed] [Google Scholar]
- Keller J.R., Jacobsen,S.E., Dubois,C.M., Hestdal,K. and Ruscetti,F.W. (1992) Transforming growth factor-β: a bidirectional regulator of hematopoietic cell growth. Int. J. Cell Cloning, 10, 2–11. [DOI] [PubMed] [Google Scholar]
- Kulkarni A.B. et al. (1993) Transforming growth factor-β1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA, 90, 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Weis F.M., Boyd F.T., Ignotz R.A. and Massagué,J. (1991) Responsiveness to transforming growth factor-β (TGF-β) restored by genetic complementation between cells defective in TGF-β receptors I and II. J. Biol. Chem., 266, 9108–9112. [PubMed] [Google Scholar]
- Letterio J.J., Geiser,A.G., Kulkarni,A.B., Roche,N.S., Sporn,M.B. and Roberts,A.B. (1994) Maternal rescue of transforming growth factor-β1 null mice. Science, 264, 1936–1938. [DOI] [PubMed] [Google Scholar]
- Li D.Y., Sorensen,L.K., Brooke,B.S., Urness,L.D., Davis,E.C., Taylor,D.G., Boak,B.B. and Wendel,D.P. (1999) Defective angiogenesis in mice lacking endoglin. Science, 284, 1534–1537. [DOI] [PubMed] [Google Scholar]
- Lux A., Attisano,L. and Marchuk,D.A. (1999) Assignment of transforming growth factor β1 and β3 and a third new ligand to the type I receptor ALK-1. J. Biol. Chem., 274, 9984–9992. [DOI] [PubMed] [Google Scholar]
- Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem., 67, 753–791. [DOI] [PubMed] [Google Scholar]
- McAllister K.A. et al. (1994) Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nature Genet., 8, 345–351. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N.L. and Wahl,S.M. (1994) Transforming growth factor-β: a matter of life and death. J. Leukoc. Biol., 55, 401–409. [DOI] [PubMed] [Google Scholar]
- Merwin J.R., Newman,W., Beall,L.D., Tucker,A. and Madri,J. (1991) Vascular cells respond differentially to transforming growth factors β1 and β2 in vitro. Am. J. Pathol., 138, 37–51. [PMC free article] [PubMed] [Google Scholar]
- Muller G., Behrens,J., Nussbaumer,U., Bohlen,P. and Birchmeier,W. (1987) Inhibitory action of transforming growth factor-β on endothelial cells. Proc. Natl Acad. Sci. USA, 84, 5600–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.P. et al. (2000) Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc. Natl Acad. Sci. USA, 97, 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M., Oshima,H. and Taketo,M.M. (1996) TGF-β receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev. Biol., 179, 297–302. [DOI] [PubMed] [Google Scholar]
- Pepper M.S. (1997) Transforming growth factor-β: vasculogenesis, angiogenesis and vessel wall integrity. Cytokine Growth Factor Rev., 8, 21–43. [DOI] [PubMed] [Google Scholar]
- Persson U., Izumi,H., Souchelnytskyi,S., Itoh,S., Grimsby,S., Engström,U., Heldin,C.-H., Funa,K. and ten Dijke,P. (1998) The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett., 434, 83–87. [DOI] [PubMed] [Google Scholar]
- Piek E., Westermark,U., Kastemar,M., Heldin,C.-H., van Zoelen,E.J., Nister,M. and ten Dijke,P. (1999) Expression of transforming-growth-factor (TGF)-β receptors and Smad proteins in glioblastoma cell lines with distinct responses to TGF-β1. Int. J. Cancer, 80, 756–763. [DOI] [PubMed] [Google Scholar]
- Proetzel G., Pawlowski,S.A., Wiles,M.V., Yin,M., Boivin,G.P., Howles,P.N., Ding,J., Ferguson,M.W. and Doetschman,T. (1995) Transforming growth factor-β3 is required for secondary palate fusion. Nature Genet., 11, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. (1997) Mechanisms of angiogenesis. Nature, 386, 671–674. [DOI] [PubMed] [Google Scholar]
- Roberts A.B. and Sporn,M.B. (1990) The transforming growth factors-β. In Sporn,M.B. and Roberts,A.B. (eds), Handbook of Experimental Pharmacology. Vol. 95. Springer Verlag, New York, NY, pp. 418–472.
- Roelen B.A., van Rooijen M.A. and Mummery C.L. (1997) Expression of ALK-1, a type 1 serine/threonine kinase receptor, coincides with sites of vasculogenesis and angiogenesis in early mouse development. Dev. Dyn., 209, 418–430. [DOI] [PubMed] [Google Scholar]
- Sanford L.P., Ormsby,I., Gittenberger-de Groot,A.C., Sariola,H., Friedman,R., Boivin,G.P., Cardell,E.L. and Doetschman,T. (1997) TGF-β2 knockout mice have multiple developmental defects that are non-overlapping with other TGF-β knockout phenotypes. Development, 124, 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull M.M. et al. (1992) Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature, 359, 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnicka E., Ruscetti,F.W., Priestley,G.V., Wolf,N.S. and Bartelmez,S.H. (1996) Transforming growth factor β1 directly and reversibly inhibits the initial cell divisions of long-term repopulating hematopoietic stem cells. Blood, 88, 82–88. [PubMed] [Google Scholar]
- Sporn M.B. and Roberts,A.B. (1988) Peptide growth factors are multifunctional. Nature, 332, 217–219. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Shioda,N., Maeda,T., Tada,M. and Ueno,N. (1994) A mouse TGF-β type I receptor that requires type II receptor for ligand binding. Biochem. Biophys. Res. Commun., 198, 1063–1069. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita,H., Ichijo,H., Franzén,P., Laiho,M., Miyazono,K. and Heldin,C.-H. (1994) Characterization of type I receptors for transforming growth factor-β and activin. Science, 264, 101–104. [DOI] [PubMed] [Google Scholar]
- Wang Y.Q., Sizeland,A., Wang,X.F. and Sassoon,D. (1995) Restricted expression of type-II TGF-β receptor in murine embryonic development suggests a central role in tissue modeling and CNS patterning. Mech. Dev., 52, 275–289. [DOI] [PubMed] [Google Scholar]
- Wieser R., Wrana,J.L. and Massagué,J. (1995) GS domain mutations that constitutively activate TβR-I, the downstream signalling component in the TGF-β receptor complex. EMBO J., 14, 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.L., Courtneidge,S.A. and Wagner,E.F. (1988) Embryonic lethalities and endothelial tumors in chimeric mice expressing polyoma virus middle T oncogene. Cell, 52, 121–131. [DOI] [PubMed] [Google Scholar]
- Wong P.M., Chung,S.W., Chui,D.H. and Eaves,C.J. (1986) Properties of the earliest clonogenic hemopoietic precursors to appear in the developing murine yolk sac. Proc. Natl Acad. Sci. USA, 83, 3851–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J.L., Attisano,L., Wieser,R., Ventura,F. and Massagué,J. (1994) Mechanism of activation of the TGF-β receptor. Nature, 370, 341–347. [DOI] [PubMed] [Google Scholar]
- Yamashita H., ten Dijke,P., Franzén,P., Miyazono,K. and Heldin,C.-H. (1994) Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-β. J. Biol. Chem., 269, 20172–20178. [PubMed] [Google Scholar]
- Yang X., Castilla,L.H., Xu,X., Li,C., Gotay,J., Weinstein,M., Liu,P.P. and Deng,C.X. (1999) Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development, 126, 1571–1580. [DOI] [PubMed] [Google Scholar]
- Yaswen L., Kulkarni,A.B., Fredrickson,T., Mittleman,B., Schiffman,R., Payne,S., Longenecker,G., Mozes,E. and Karlsson,S. (1996) Autoimmune manifestations in the transforming growth factor β1 knockout mouse. Blood, 87, 1439–1445. [PubMed] [Google Scholar]
- Zwijsen A., Goumans,M.J., Lawson,K.A., van Rooijen,M.A. and Mummery,C.L. (1999) Ectopic expression of the transforming growth factor-β type II receptor disrupts mesoderm organization during mouse gastrulation. Dev. Dyn., 214, 141–151. [DOI] [PubMed] [Google Scholar]