Abstract
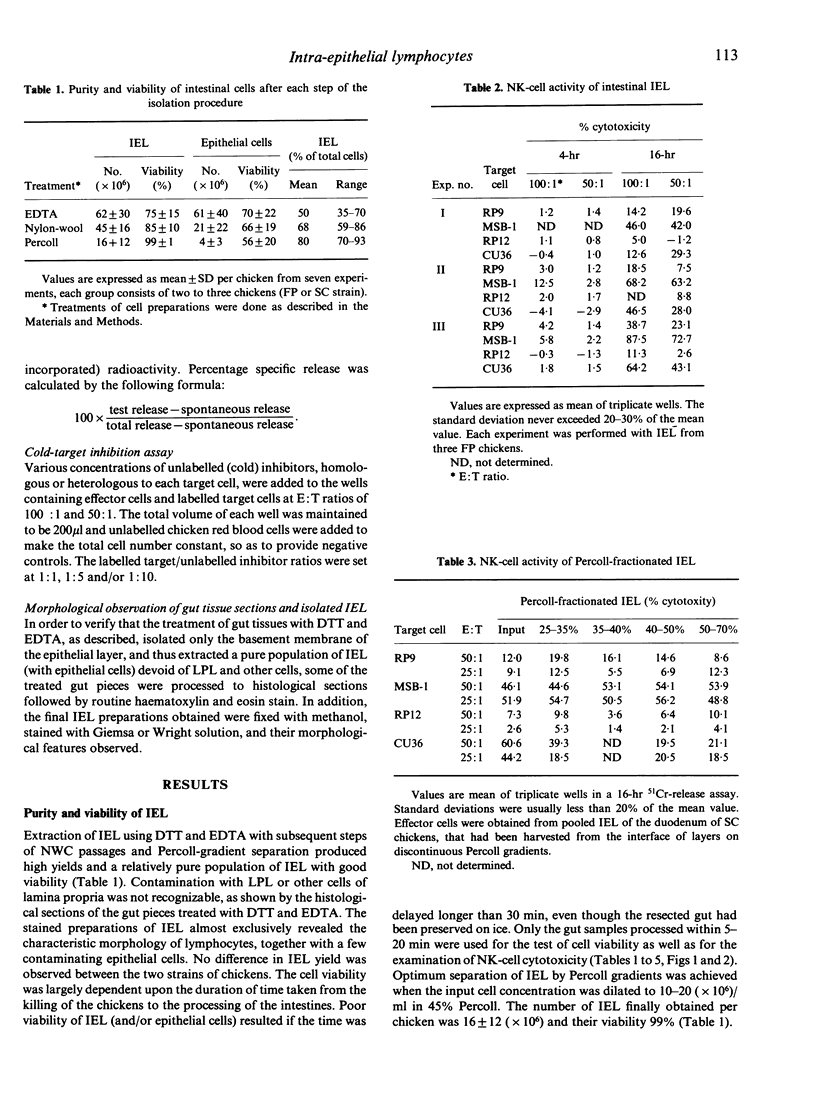
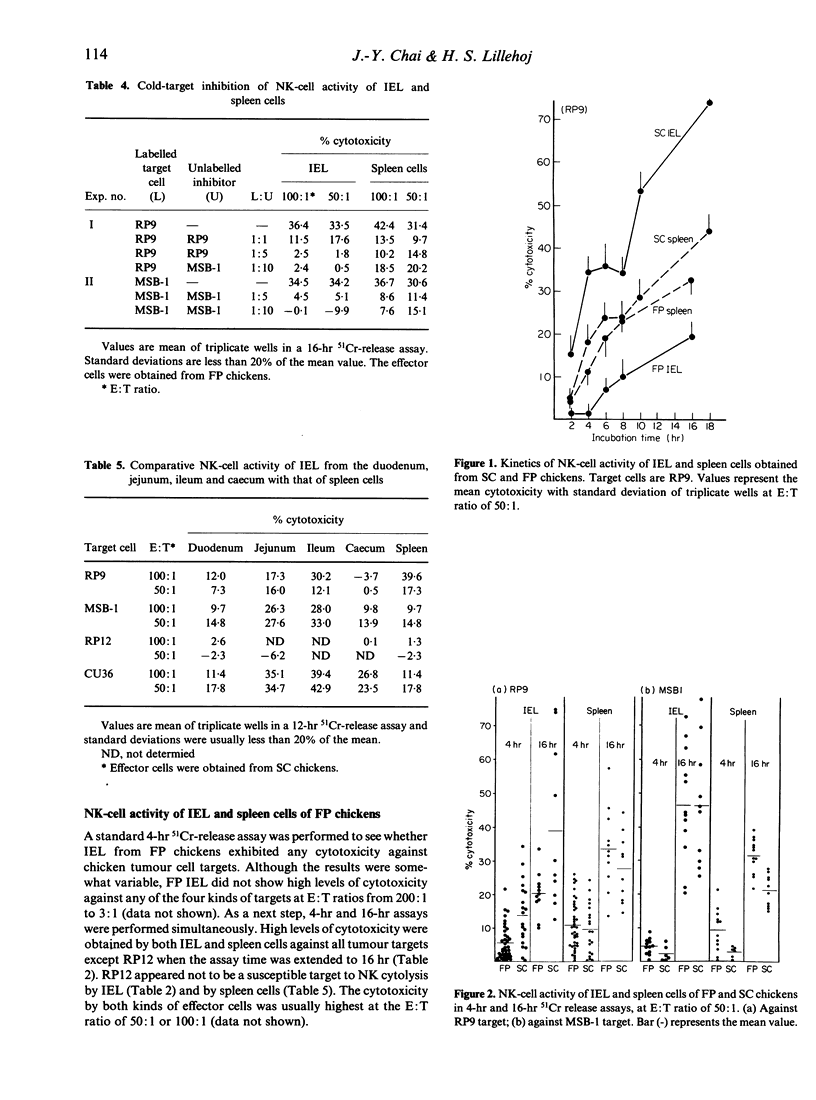
Intestinal intra-epithelial lymphocytes (IEL) of SC or FP chickens were isolated and examined for their natural killer (NK)-cell activity against chicken tumour cell lines, LSCC-RP9 (RP9), LSCC-RP12 (RP12), MDCC-MSB-1 (MSB-1) and MDCC-CU36 (CU36). In general, IEL of satisfactory yield and of good viability were obtained with EDTA treatment of the gut tissues, followed by rapid passages of the resultant cells through nylon-wool columns and centrifugation on two-step Percoll density gradients (45% and 80%). In 4-hr and 16-hr 51Cr-release assays, the NK-cell activity of chicken IEL depended not only upon the type of target cells but also upon the incubation time and the host genetic background. RP9, MSB-1 and CU36 were susceptible to NK lysis by IEL and by spleen cells, while RP12 was resistant to lysis even after a prolonged incubation. In kinetic studies the cytotoxicity was detactable from 2 hr after incubation and progressively increased up to 16 or 18 hr. The IEL of SC chickens revealed significantly higher levels of NK-cell activity against RP9 than FP-strain chickens, whereas their splenic NK-cell activity was not significantly different. Against MSB-1 targets, however, IEL of SC and FP chickens showed similar levels of NK-cell activity while their spleens did not (being higher in FP). When tested in FP chickens, IEL NK-cell activity was inhibited by the addition of unlabelled homologous target cells. In general, NK-cell activity was higher in the jejunum and ileum than in the duodenum and caecum. Efforts to enrich IEL NK-effector cells by discontinuous Percoll gradients were not successful. The results of the present study show that IEL of chicken intestine contain effector cells that can mediate NK-cell activity against chicken tumour cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud-Battandier F., Bundy B. M., O'Neill M., Bienenstock J., Nelson D. L. Cytotoxic activities of gut mucosal lymphoid cells in guinea pigs. J Immunol. 1978 Sep;121(3):1059–1065. [PubMed] [Google Scholar]
- Arnaud-Battandier F., Lawrence E. C., Blaese R. M. Lymphoid populations of gut mucosa in chickens. Dig Dis Sci. 1980 Apr;25(4):252–259. doi: 10.1007/BF01308514. [DOI] [PubMed] [Google Scholar]
- Bland P. W., Richens E. R., Britton D. C., Lloyd J. V. Isolation and purification of human large bowel mucosal lymphoid cells: effect of separation technique on functional characteristics. Gut. 1979 Dec;20(12):1037–1046. doi: 10.1136/gut.20.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C. G. Reversible induction of natural killer cell activity in cloned murine cytotoxic T lymphocytes. Nature. 1983 Sep 8;305(5930):155–158. doi: 10.1038/305155a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Carman P. S., Ernst P. B., Rosenthal K. L., Clark D. A., Befus A. D., Bienenstock J. Intraepithelial leukocytes contain a unique subpopulation of NK-like cytotoxic cells active in the defense of gut epithelium to enteric murine coronavirus. J Immunol. 1986 Mar 1;136(5):1548–1553. [PubMed] [Google Scholar]
- Chiba M., Bartnik W., ReMine S. G., Thayer W. R., Shorter R. G. Human colonic intraepithelial and lamina proprial lymphocytes: cytotoxicity in vitro and the potential effects of the isolation method on their functional properties. Gut. 1981 Mar;22(3):177–186. doi: 10.1136/gut.22.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. D., Parrott D. M. Cytotoxic T cells in small intestine epithelial, lamina propria and lung lymphocytes. Immunology. 1981 Oct;44(2):367–371. [PMC free article] [PubMed] [Google Scholar]
- Dillon S. B., MacDonald T. T. Functional properties of lymphocytes isolated from murine small intestinal epithelium. Immunology. 1984 Jul;52(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Kagnoff M. F., Fiocchi C., Befus A. D., Targan S. Intestinal immunity and inflammation: recent progress. Gastroenterology. 1986 Sep;91(3):746–768. doi: 10.1016/0016-5085(86)90649-9. [DOI] [PubMed] [Google Scholar]
- Ferguson A. Intraepithelial lymphocytes of the small intestine. Gut. 1977 Nov;18(11):921–937. doi: 10.1136/gut.18.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B. Effector cells in avian spontaneous and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1980 Sep;125(3):1161–1166. [PubMed] [Google Scholar]
- Flexman J. P., Shellam G. R., Mayrhofer G. Natural cytotoxicity, responsiveness to interferon and morphology of intra-epithelial lymphocytes from the small intestine of the rat. Immunology. 1983 Apr;48(4):733–741. [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B. Natural killer (NK) cells and their possible roles in resistance against disease. Clin Immunol Rev. 1981;1(1):1–65. [PubMed] [Google Scholar]
- Lam K. M., Linna T. J. Transfer of natural resistance to Marek's disease (JMV) with nonimmune spleen cells. II. Further characterization of protecting cell population. J Immunol. 1980 Aug;125(2):715–718. [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H., Hackett J., Jr, Tutt M., Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986 Nov 1;137(9):2735–2739. [PubMed] [Google Scholar]
- Lawn A. M., Rose M. E. Mucosal transport of Eimeria tenella in the cecum of the chicken. J Parasitol. 1982 Dec;68(6):1117–1123. [PubMed] [Google Scholar]
- Leibold W., Janotte G., Peter H. H. Spontaneous cell-mediated cytotoxicity (SCMC) in various mammalian species and chickens: selective reaction pattern and different mechanisms. Scand J Immunol. 1980;11(2):203–222. doi: 10.1111/j.1365-3083.1980.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S., Ruff M. D. Comparison of disease susceptibility and subclass-specific antibody response in SC and FP chickens experimentally inoculated with Eimeria tenella, E. acervulina, or E. maxima. Avian Dis. 1987 Jan-Mar;31(1):112–119. [PubMed] [Google Scholar]
- Malkovský M., Jíra M., Madar J., Malkovska V., Loveland B., Asherson G. L. Generation of lymphokine-activated killer cells does not require DNA synthesis. Immunology. 1987 Mar;60(3):471–473. [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Whately R. J. Granular intraepithelial lymphocytes of the rat small intestine. I. Isolation, presence in T lymphocyte-deficient rats and bone marrow origin. Int Arch Allergy Appl Immunol. 1983;71(4):317–327. doi: 10.1159/000233414. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Tait R. C., MacKenzie S., Davies M. D., Parrott D. M. Analysis of natural killer effector and suppressor activity by intraepithelial lymphocytes from mouse small intestine. Clin Exp Immunol. 1983 Apr;52(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Mándi Y., Seprényi G., Pusztai R., Béládi I. Are granulocytes the main effector cells of natural cytotoxicity in chickens? Immunobiology. 1985 Nov;170(4):284–292. doi: 10.1016/S0171-2985(85)80077-2. [DOI] [PubMed] [Google Scholar]
- Nauss K. M., Pavlina T. M., Kumar V., Newberne P. M. Functional characteristics of lymphocytes isolated from the rat large intestine. Response to T-cell mitogens and natural killer cell activity. Gastroenterology. 1984 Mar;86(3):468–475. [PubMed] [Google Scholar]
- Nencioni L., Villa L., Boraschi D., Berti B., Tagliabue A. Natural and antibody-dependent cell-mediated activity against Salmonella typhimurium by peripheral and intestinal lymphoid cells in mice. J Immunol. 1983 Feb;130(2):903–907. [PubMed] [Google Scholar]
- Rosenberg S. Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J Natl Cancer Inst. 1985 Oct;75(4):595–603. [PubMed] [Google Scholar]
- Sharma J. M., Coulson B. D. Presence of natural killer cells in specific-pathogen-free chickens. J Natl Cancer Inst. 1979 Aug;63(2):527–531. [PubMed] [Google Scholar]
- Sharma J. M., Okazaki W. Natural killer cell activity in chickens: target cell analysis and effect of antithymocyte serum on effector cells. Infect Immun. 1981 Mar;31(3):1078–1085. doi: 10.1128/iai.31.3.1078-1085.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A., Befus A. D., Clark D. A., Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982 Jun 1;155(6):1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Haspel M. V., Parker D. C., Holmes K. V. Natural cytotoxicity against mouse hepatitis virus-infected cells. II. A cytotoxic effector cell with a B lymphocyte phenotype. J Immunol. 1986 Feb 15;136(4):1454–1460. [PubMed] [Google Scholar]
- Wilson A. D., Stokes C. R., Bourne F. J. Morphology and functional characteristics of isolated porcine intraepithelial lymphocytes. Immunology. 1986 Sep;59(1):109–113. [PMC free article] [PubMed] [Google Scholar]


