Abstract
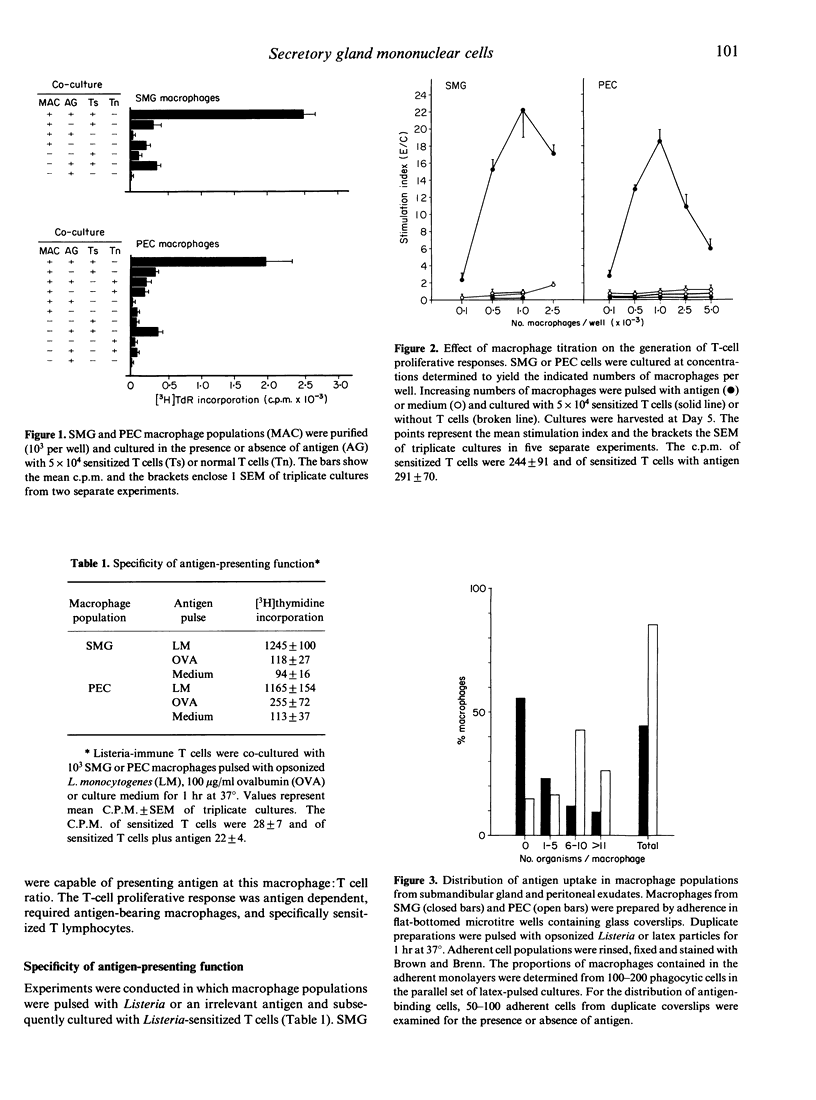
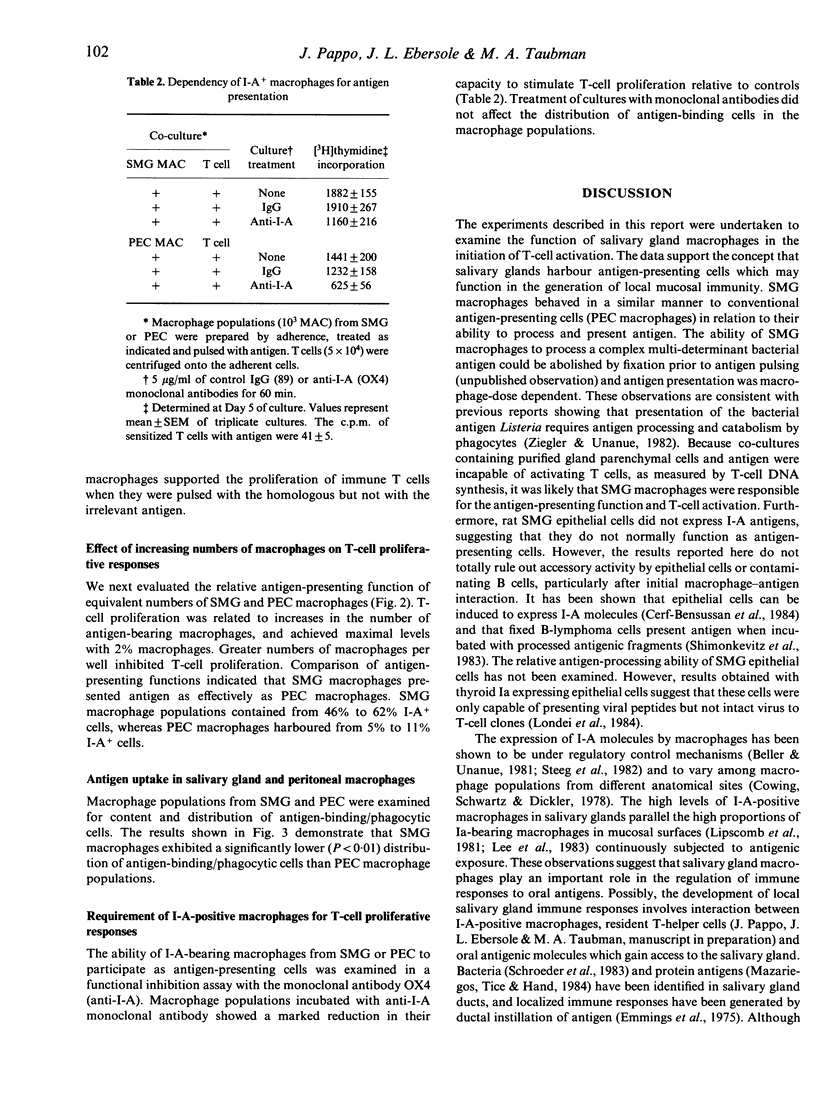
The function of salivary gland macrophages in the induction of local immunity in secretory organs was investigated in Fischer 344 rats. Macrophages obtained from dispersed submandibular gland (SMG) cells were characterized and examined for their ability to present antigen to T cells. Populations of SMG-adherent cells contained approximately 80% macrophages, of which 46-62% were I-A+ cells. These numbers were from five to 10-fold greater than the I-A+ cells in macrophage populations from peritoneal exudates (5-11%). SMG macrophages functioned effectively as antigen-presenting cells. Antigen presentation was antigen specific, macrophage dose dependent and inhibitable by monoclonal anti-I-A antibodies. These studies suggest that a functional salivary-gland immune-response pathway exists that can function independently of a gut-associated lymphocyte-homing mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Unanue E. R. Regulation of macrophage populations. II. Synthesis and expression of Ia antigens by peritoneal exudate macrophages is a transient event. J Immunol. 1981 Jan;126(1):263–269. [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Cowing C., Schwartz B. D., Dickler H. B. Macrophage Ia antigens. I. macrophage populations differ in their expression of Ia antigens. J Immunol. 1978 Feb;120(2):378–384. [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Rabbit Peyer's patches, appendix, and popliteal lymph node B lymphocytes: a comparative analysis of their membrane immunoglobulin components and plasma cell precursor potential. J Immunol. 1975 Jan;114(1 Pt 2):492–502. [PubMed] [Google Scholar]
- Ebersole J. L., Frey D. E., Taubman M. A., Smith D. J. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J Periodontal Res. 1980 Nov;15(6):621–632. doi: 10.1111/j.1600-0765.1980.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Emmings F. G., Evans R. T., Genco R. J. Antibody response in the parotid fluid and serum of Irus monkeys (Macaca fascicularis) after local immunization with Streptococcus mutans. Infect Immun. 1975 Aug;12(2):281–292. doi: 10.1128/iai.12.2.281-292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- GORLIN R. J. Heterotopic lymphoid tissue; a diagnostic problem. Oral Surg Oral Med Oral Pathol. 1957 Jan;10(1):87–89. doi: 10.1016/s0030-4220(57)80119-4. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Cebra J. J. Differentiated B lymphocytes. Potential to express particular antibody variable and constant regions depends on site of lymphoid tissue and antigen load. J Exp Med. 1979 Jan 1;149(1):216–227. doi: 10.1084/jem.149.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburg S. I., Manejias R. E., Rabinovitch M. Macrophage activation: increased ingestion of IgG-coated erythrocytes after administration of interferon inducers to mice. J Exp Med. 1978 Feb 1;147(2):593–598. doi: 10.1084/jem.147.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. E., Lally E. T., Nakamura M. C., Montgomery P. C. Migration of IgA-bearing lymphocytes into salivary glands. Cell Immunol. 1981 Sep 1;63(1):203–209. doi: 10.1016/0008-8749(81)90042-3. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Lipscomb M. F., Toews G. B., Lyons C. R., Uhr J. W. Antigen presentation by guinea pig alveolar macrophages. J Immunol. 1981 Jan;126(1):286–291. [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazariegos M. R., Tice L. W., Hand A. R. Alteration of tight junctional permeability in the rat parotid gland after isoproterenol stimulation. J Cell Biol. 1984 May;98(5):1865–1877. doi: 10.1083/jcb.98.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Mestecky J., Arnold R. R., Bozzo L. Ingestion of Streptococcus mutans induces secretory immunoglobulin A and caries immunity. Science. 1976 Jun 18;192(4245):1238–1240. doi: 10.1126/science.1273589. [DOI] [PubMed] [Google Scholar]
- Nair P. N., Schroeder H. E. Local immune response to repeated topical antigen application in the simian labial mucosa. Infect Immun. 1983 Jul;41(1):399–409. doi: 10.1128/iai.41.1.399-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P. N., Schroeder H. E. Retrograde access of antigens to the minor salivary glands in the monkey Macaca fascicularis. Arch Oral Biol. 1983;28(2):145–152. doi: 10.1016/0003-9969(83)90121-8. [DOI] [PubMed] [Google Scholar]
- Reynolds C. W., Sharrow S. O., Ortaldo J. R., Herberman R. B. Natural killer activity in the rat. II. Analysis of surface antigens on LGL by flow cytometry. J Immunol. 1981 Dec;127(6):2204–2208. [PubMed] [Google Scholar]
- Schroeder H. E., Moreillon M. C., Nair P. N. Architecture of minor salivary gland duct/lymphoid follicle associations and possible antigen-recognition sites in the monkey Macaca fascicularis. Arch Oral Biol. 1983;28(2):133–143. doi: 10.1016/0003-9969(83)90120-6. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


