Abstract
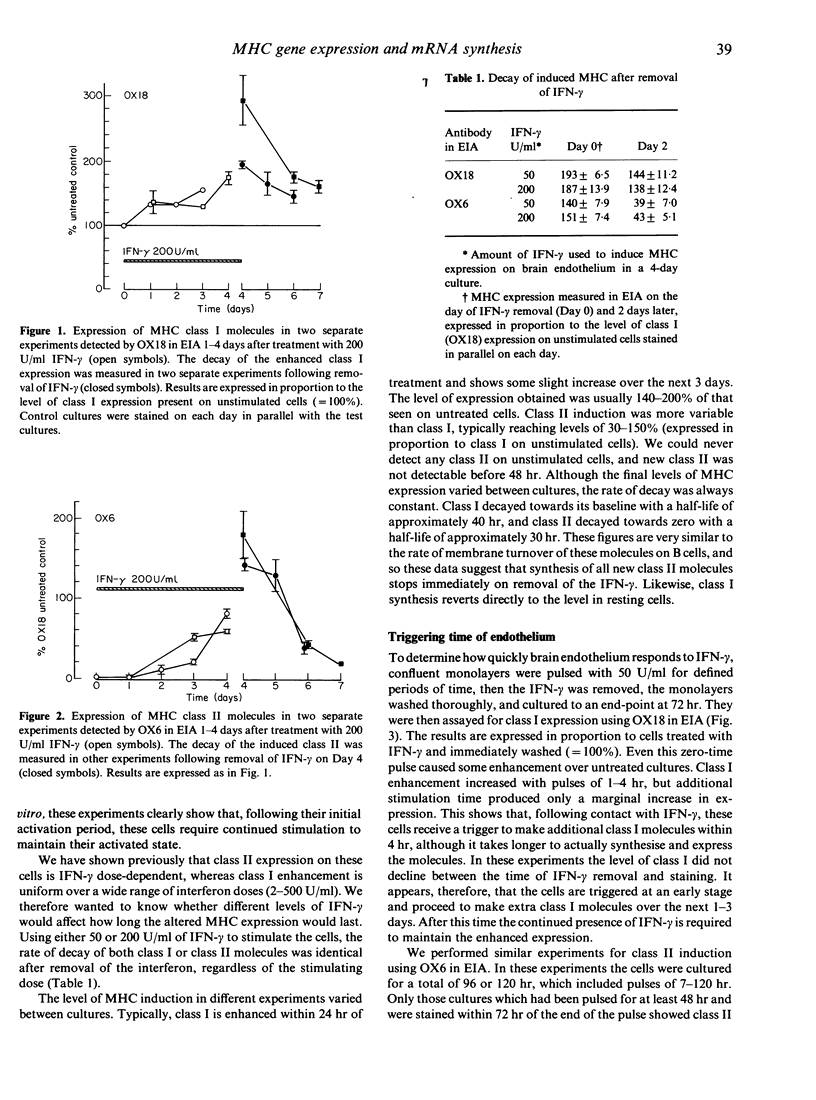
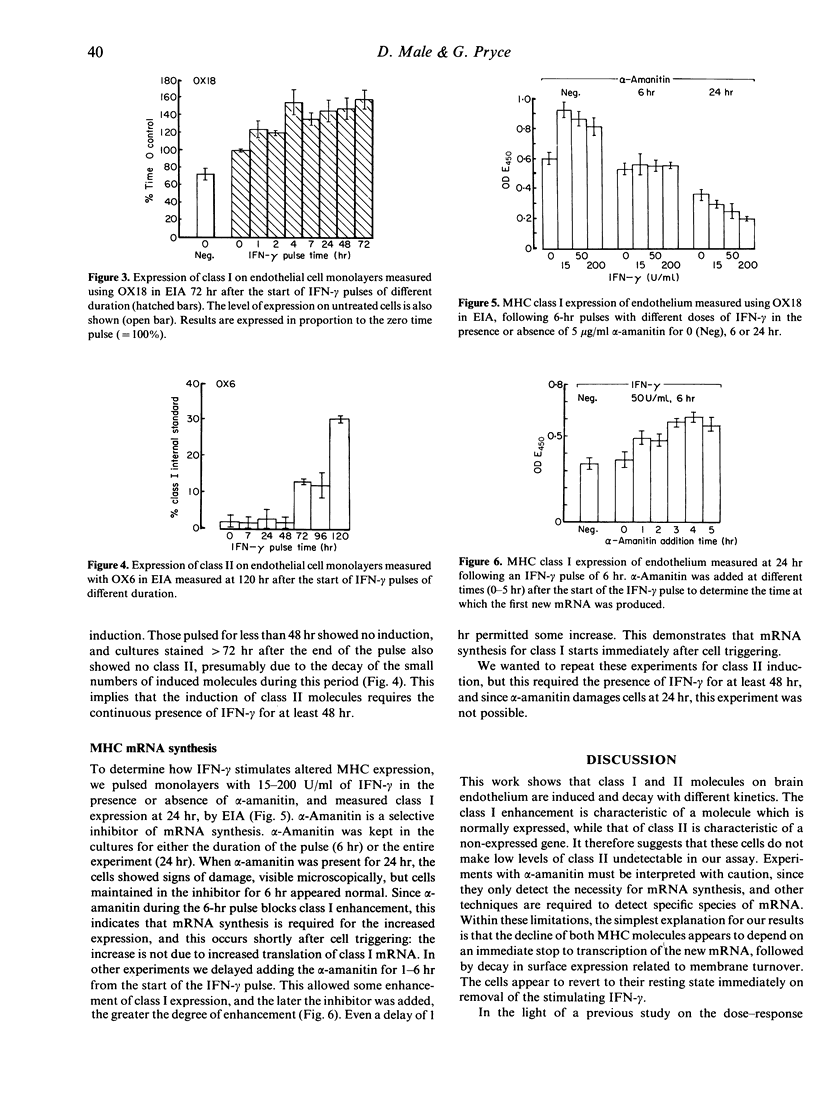
Rat brain endothelium was examined in vitro to determine the sequence of events in MHC gene activation following IFN-gamma stimulation. The cell-triggering time, kinetics of mRNA synthesis, rate of MHC induction and rate of decay were measured by quantifying cell-surface MHC expression in the presence or absence of alpha-amanitin. Enhanced class I expression is triggered immediately after IFN-gamma stimulation, and is maximally induced by 4 hr of stimulation. New class I mRNA synthesis starts immediately and proceeds over the next 24 hr. This is followed by increased expression of class I molecules, which reaches plateau levels by 24 hr. While IFN-gamma is present, enhanced class I expression is maintained at 140-200% of that seen on resting cells. On removal of IFN-gamma, class I expression decays towards the levels seen on resting cells, with a half-life of approximately 40 hr. Class II molecules can be induced on these cells as well, but it requires the continuous presence of higher levels of IFN-gamma for more than 48 hr to trigger the cells. Induced class II molecules start to appear 2 days after pulsing and continue to increase until Day 4. If the IFN-gamma is removed from the cultures, class II expression declines rapidly towards zero, with a half-life of approximately 30 hr.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Craggs R. I., Webster H. D. Ia antigens in the normal rat nervous system and in lesions of experimental allergic encephalomyelitis. Acta Neuropathol. 1985;68(4):263–272. doi: 10.1007/BF00690828. [DOI] [PubMed] [Google Scholar]
- Fontana A., Fierz W. The endothelium--astrocyte immune control system of the brain. Springer Semin Immunopathol. 1985;8(1-2):57–70. doi: 10.1007/BF00197247. [DOI] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Giulian D., Baker T. J., Shih L. C., Lachman L. B. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986 Aug 1;164(2):594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. W., Betz A. L., Bowman P. D. Use of isolated brain capillaries and cultured endothelial cells to study the blood-brain barrier. Fed Proc. 1984 Feb;43(2):191–195. [PubMed] [Google Scholar]
- Hickey W. F., Gonatas N. K., Kimura H., Wilson D. B. Identification and quantitation of T lymphocyte subsets found in the spinal cord of the Lewis rat during acute experimental allergic encephalomyelitis. J Immunol. 1983 Dec;131(6):2805–2809. [PubMed] [Google Scholar]
- Hughes C. C., Lantos P. L. Brain capillary endothelial cells in vitro lack surface IgG Fc receptors. Neurosci Lett. 1986 Jul 11;68(1):100–106. doi: 10.1016/0304-3940(86)90237-5. [DOI] [PubMed] [Google Scholar]
- Male D. K., Pryce G., Hughes C. C. Antigen presentation in brain: MHC induction on brain endothelium and astrocytes compared. Immunology. 1987 Mar;60(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- McCarron R. M., Kempski O., Spatz M., McFarlin D. E. Presentation of myelin basic protein by murine cerebral vascular endothelial cells. J Immunol. 1985 May;134(5):3100–3103. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Tabira T., Endoh M., Steinman L. Ia expression in chronic relapsing experimental allergic encephalomyelitis induced by long-term cultured T cell lines in mice. Lab Invest. 1986 Mar;54(3):345–352. [PubMed] [Google Scholar]
- Sedgwick J. D., Mason D. W. The mechanism of inhibition of experimental allergic encephalomyelitis in the rat by monoclonal antibody against CD4. J Neuroimmunol. 1986 Dec;13(2):217–232. doi: 10.1016/0165-5728(86)90066-4. [DOI] [PubMed] [Google Scholar]
- Sobel R. A., Blanchette B. W., Bhan A. K., Colvin R. B. The immunopathology of experimental allergic encephalomyelitis. II. Endothelial cell Ia increases prior to inflammatory cell infiltration. J Immunol. 1984 May;132(5):2402–2407. [PubMed] [Google Scholar]
- Traugott U., Raine C. S., McFarlin D. E. Acute experimental allergic encephalomyelitis in the mouse: immunopathology of the developing lesion. Cell Immunol. 1985 Mar;91(1):240–254. doi: 10.1016/0008-8749(85)90047-4. [DOI] [PubMed] [Google Scholar]
- Traugott U., Scheinberg L. C., Raine C. S. On the presence of Ia-positive endothelial cells and astrocytes in multiple sclerosis lesions and its relevance to antigen presentation. J Neuroimmunol. 1985 Apr;8(1):1–14. doi: 10.1016/s0165-5728(85)80043-6. [DOI] [PubMed] [Google Scholar]
- Vass K., Lassmann H., Wekerle H., Wisniewski H. M. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol. 1986;70(2):149–160. doi: 10.1007/BF00691433. [DOI] [PubMed] [Google Scholar]
- Vass K., Lassmann H., Wisniewski H. M., Iqbal K. Ultracytochemical distribution of myelin basic protein after injection into the cerebrospinal fluid. Evidence for transport through the blood-brain barrier and binding to the luminal surface of cerebral veins. J Neurol Sci. 1984 Mar;63(3):423–433. doi: 10.1016/0022-510x(84)90165-5. [DOI] [PubMed] [Google Scholar]
- Wagner C. R., Vetto R. M., Burger D. R. The mechanism of antigen presentation by endothelial cells. Immunobiology. 1984 Dec;168(3-5):453–469. doi: 10.1016/S0171-2985(84)80130-8. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Sjollema P., Danta G. Immunoregulation of passively induced allergic encephalomyelitis. J Immunol. 1986 Mar 1;136(5):1676–1679. [PubMed] [Google Scholar]
- Yu C. L., Haskard D. O., Cavender D., Johnson A. R., Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985 Dec;62(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- van der Meide P. H., Dubbeld M., Vijverberg K., Kos T., Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986 Jun;67(Pt 6):1059–1071. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]


