Abstract
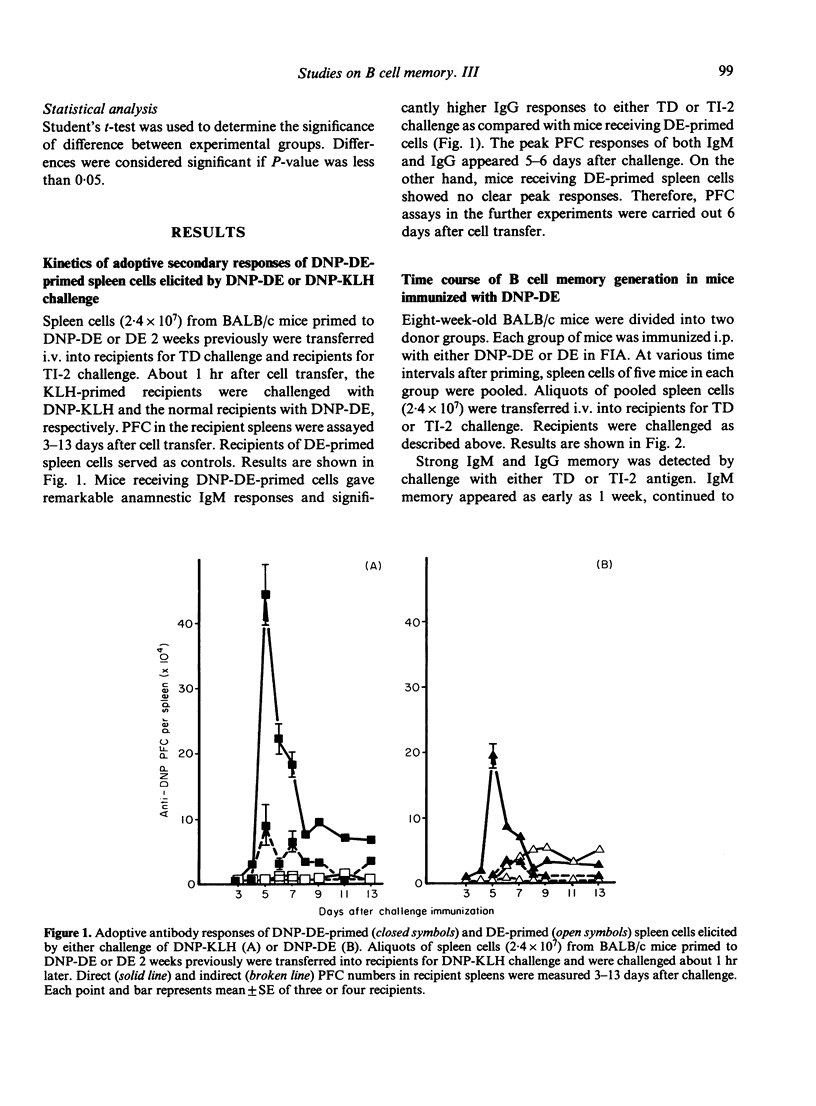
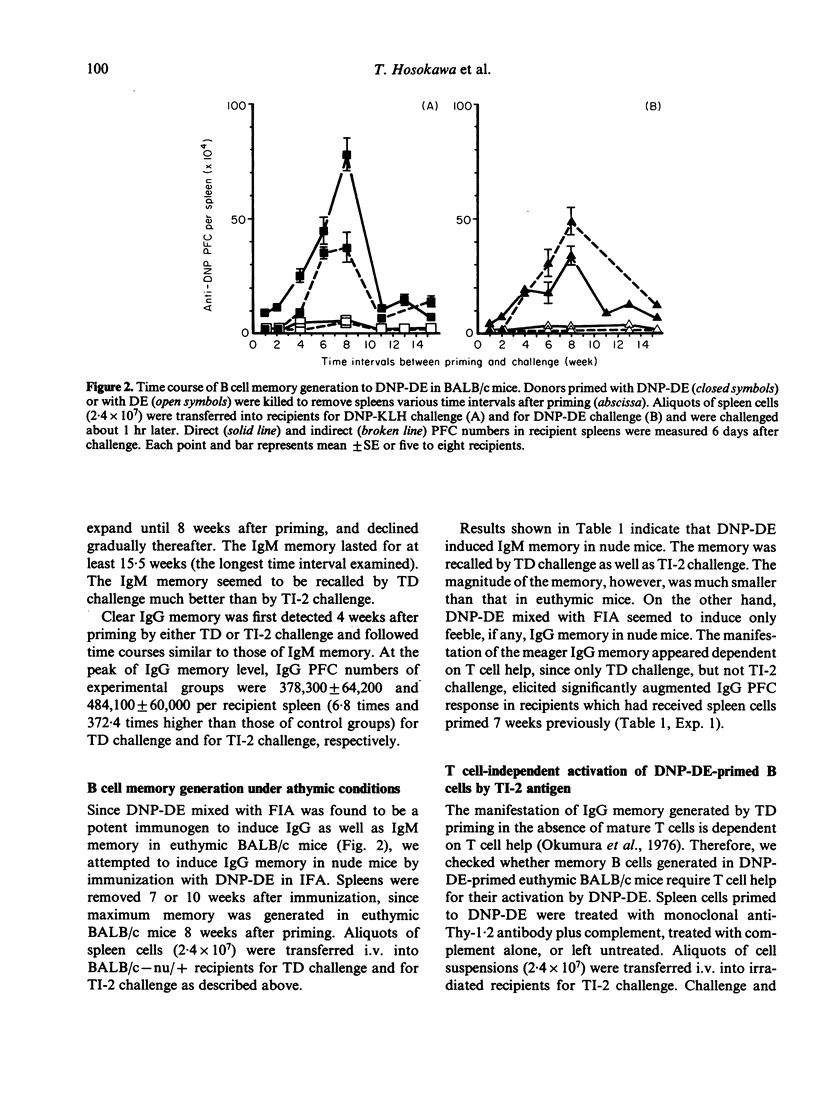
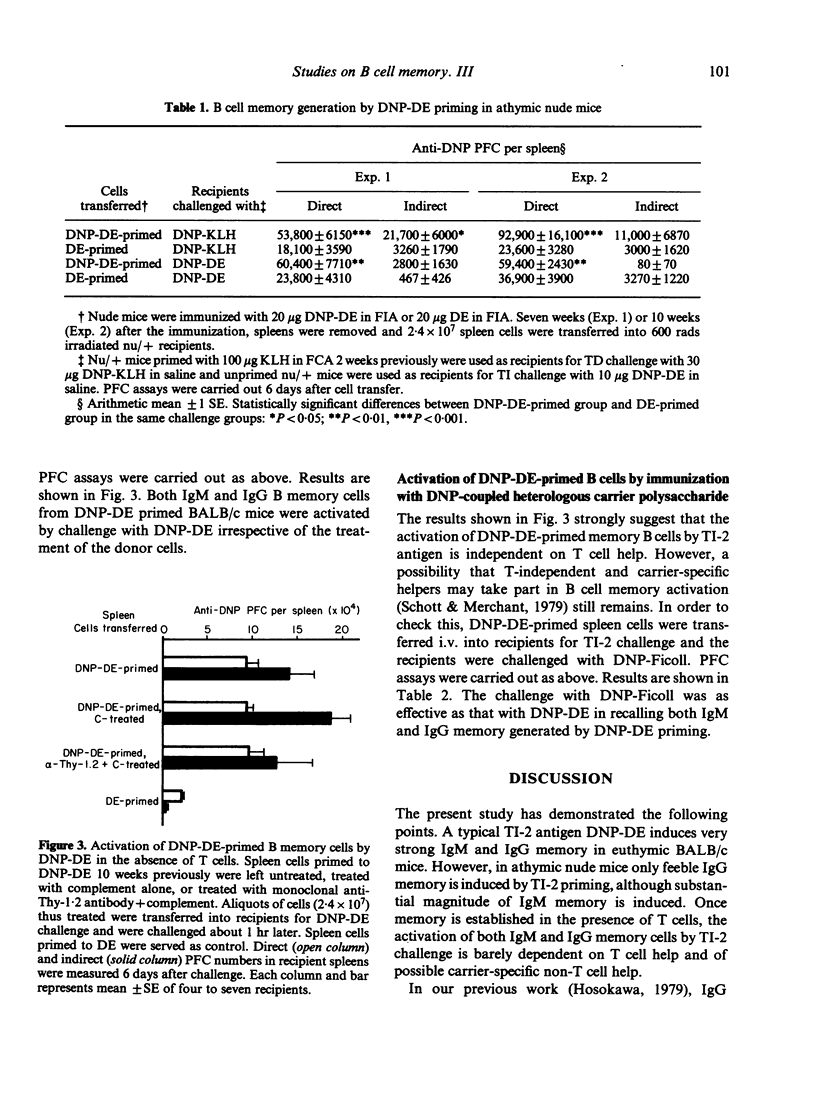
The time course of B-cell memory development to a dinitrophenyl (DNP) T-independent type-2 (TI-2) antigen was investigated by adoptive cell transfer. Strong IgM and IgG memory developed in BALB/c mice after immunization with DNP-dextran, to be recalled by challenge with either T-dependent (TD) antigen or TI-2 antigen. However, only weak IgM memory and very feeble IgG memory were detected in athymic nude mice receiving the same immunization as euthymic mice. Once memory was established under probable T cell influence, its recall by TI-2 antigen challenge seemed independent of T cell help and did not require sharing of carriers between priming and challenge antigens. The following may be concluded. (i) Long-term IgM and IgG memory is induced by TI-2 antigen priming in the presence of functional T cells. (ii) The class switch from IgM to IgG in the memory B cell pool is driven effectively by TI-2 antigen and is probably T cell-dependent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Blomgren H. T-cell response to polyvinyl pyrrolidone is linked to maturity of B cells. Nature. 1975 Feb 6;253(5491):476–477. doi: 10.1038/253476a0. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Immunocompetence of transferred thymus-marrow cell combinations. J Immunol. 1966 Dec;97(6):828–832. [PubMed] [Google Scholar]
- Colle J. H., Motta I., Truffa-Bachi P. Generation of immune memory by haptenated derivatives of thymus-independent antigens in C57BL/6 mice. I. The differentiation of memory B lymphocytes into antibody-secreting cells depends on the nature of the thymus-independent carrier used for memory induction and/or revelation. Cell Immunol. 1983 Jan;75(1):52–62. doi: 10.1016/0008-8749(83)90304-0. [DOI] [PubMed] [Google Scholar]
- Del Guercio P., Thobie N., Poirier M. F. IgM anamnestic immune response to the haptenic determinant DNP on a thymus-independent carrier. J Immunol. 1974 Jan;112(1):427–429. [PubMed] [Google Scholar]
- Diaz-Espada F., Martinez-Alonso C., Bernabe R. R. Development of B cell subsets: effect of priming on in vitro TNP-LPS responsiveness. J Immunol. 1978 Jul;121(1):13–18. [PubMed] [Google Scholar]
- EISEN H. N., KERN M., NEWTON W. T., HELMREICH E. A study of the distribution of 2,4-dinitrobenzene sensitizers between isolated lymph node cells and extracellular medium in relation to induction of contact skin sensitivity. J Exp Med. 1959 Aug 1;110(2):187–206. doi: 10.1084/jem.110.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T., Amagai T., Muramatsu S. Studies on B-cell memory. I. Generation and exhaustion of B-cell memory by thymus-dependent antigen in T-cell depleted mice. Immunology. 1979 Oct;38(2):283–289. [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T. Studies on B-cell memory. II. T-cell independent antigen can induce B-cell memory. Immunology. 1979 Oct;38(2):291–299. [PMC free article] [PubMed] [Google Scholar]
- Hosono M., Muramatsu S. Use of 2-mercaptoethanol for distinguishing between IgM and IgG antibody-producing cells of mice immunized with bovine globulin. J Immunol. 1972 Oct;109(4):857–863. [PubMed] [Google Scholar]
- Kataoka Y., Sado T. The radiosensitivity of T and B lymphocytes in mice. Immunology. 1975 Jul;29(1):121–130. [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongini P. K., Stein K. E., Paul W. E. T cell regulation of IgG subclass antibody production in response to T-independent antigens. J Exp Med. 1981 Jan 1;153(1):1–12. doi: 10.1084/jem.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Metzler C. M., Tsu T. T., Herzenberg L. A., Herzenberg L. A. Two stages of B-cell memory development with different T-cell requirements. J Exp Med. 1976 Aug 1;144(2):345–357. doi: 10.1084/jem.144.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Schott C. F., Merchant B. Carrier-specific immune memory to a thymus-independent antigen in congenitally athymic mice. J Immunol. 1979 May;122(5):1710–1718. [PubMed] [Google Scholar]
- Schrader J. W. The role of T cells in IgG production; thymus-dependent antigens induce B cell memory in the absence of T cells. J Immunol. 1975 Jun;114(6):1665–1669. [PubMed] [Google Scholar]
- Umetsu D. T., Chapman-Alexander J. M., Thorbecke G. J. Cross-priming of murine B cells with TNP conjugates of hemocyanin and Ficoll: characteristics of primed B cells responding to both antigens. J Immunol. 1979 Jul;123(1):396–404. [PubMed] [Google Scholar]


